Figure 5.
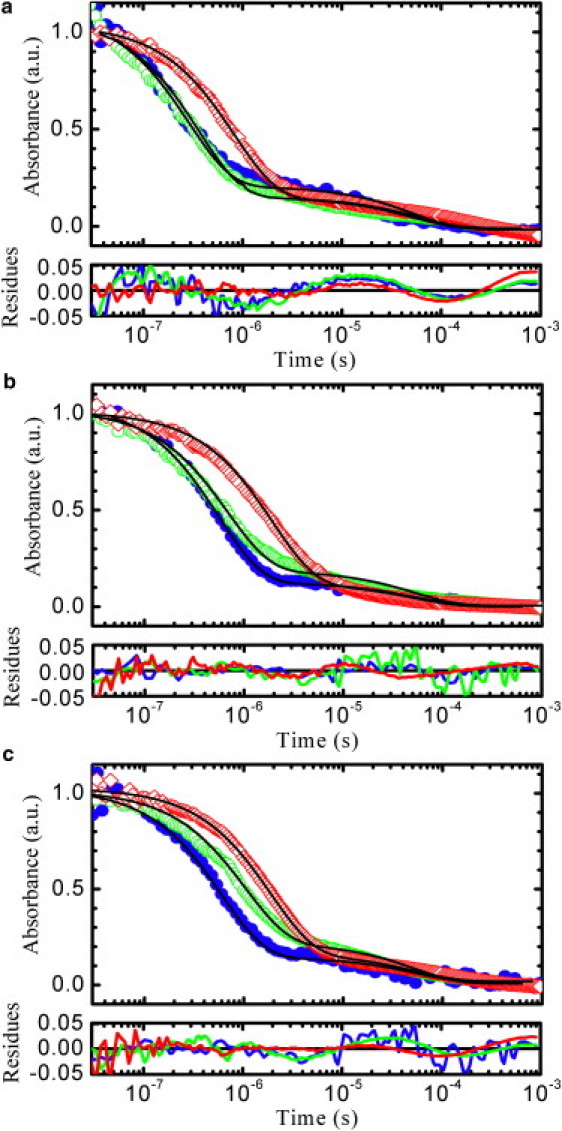
Decays of the triplet state of tryptophan. The measured absorbance decays at 20°C for the sequences agq (solid circles, blue online), age (open circles, green online), and aqe (open diamonds, red online) are shown for three solvent conditions with similar viscosities: (a) aqueous buffer with 20% of sucrose (η = 1.97 cP), (b) 8 M urea (η = 1.75 cP), and (c) 6 M GdmCl (η = 1.81 cP). The black lines represent the fits obtained with two exponential relaxations, with a fixed rate of 19 ms−1 for the slower decay (see Materials and Methods). The faster relaxation, in the range 0.1–5 μs, represents the quenching process due to tryptophan-cysteine contact formation and accounts for more than 80% of the total amplitude. To facilitate the qualitative comparison of the decay rates the absorbance amplitudes have been normalized. Typical values of absorbance measured 30 ns after the UV pulse for 50 μM peptide solutions are in the range 8–12 mOD. The residuals obtained from the fits are displayed at the bottom of each panel using the same color code of the decay curves.
