Figure 1.
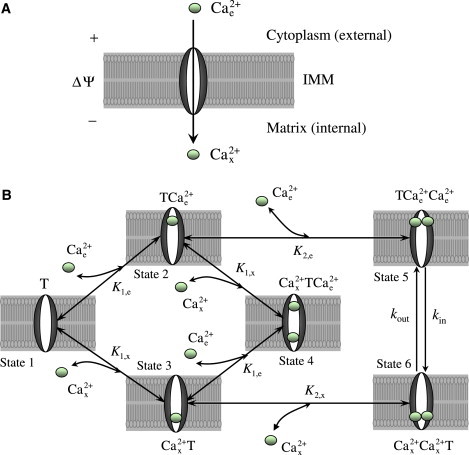
The proposed six-state kinetic mechanism of Ca2+ transport into mitochondria via the Ca2+ uniporter. The uniporter is assumed to have two binding sites for Ca2+ and the Ca2+ is assumed to bind to the uniporter from either side of the IMM. The ionized free Ca2+ from the cytoplasmic (external) side of the IMM cooperatively binds to the unbound uniporter (T) (State 1) in two steps to form the complex (State 5) which then undergo conformal changes (or flips upside down) to form the complex (State 6). The complex in the matrix (internal) side of the IMM goes through the reverse process where it dissociates in two steps to form the unbound uniporter (T) and ionized free Ca2+. The model also assumes the cross-interactions between the uniporter, external Ca2+, and internal Ca2+ to form the intermediate complex (dead end, State 4). The other two states of the uniporter are the bound uniporter (State 2) and (State 3). (K1,e, K1,x) and (K2,e, K2,x) are the two pairs of dissociation (binding) constants for the two step uniporter binding reactions with the external and internal Ca2+. The transport of Ca2+ via the Ca2+ uniporter is limited by the rate constants kin and kout which are dependent on the membrane potential ΔΨ.
