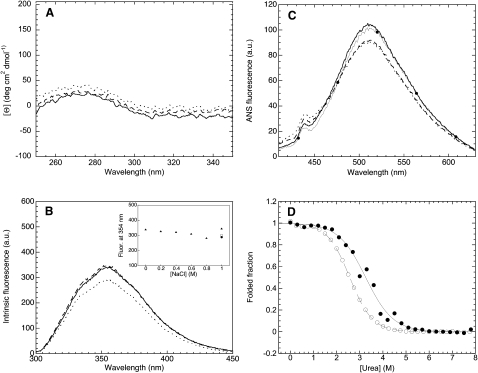
Figure 6.
(A) Near-UV CD spectra of 0.2 mg mL−1 PDZ2 in 20 mM sodium phosphate, pH 7.0, 25°C (solid line), 20 mM sodium phosphate, 1 M NaCl, pH 7.0, 25°C, before starting agitation (dashed line), and in 20 mM sodium phosphate, 1 M NaCl, pH 7.0, 25°C, after 3 h agitation at 37°C (dotted line). (B) Intrinsic fluorescence spectra of 0.4 mg mL−1 PDZ2 in 20 mM sodium phosphate, pH 7.0, 25°C (solid line), 20 mM sodium phosphate, 1 M NaCl, pH 7.0, 25°C, before starting aggregation (dashed line), and in 20 mM sodium phosphate, 1 M NaCl, pH 7.0, 25°C, after 3 h agitation at 37°C (dotted line). The inset shows the intrinsic fluorescence at 354 nm versus NaCl concentration before agitation (solid triangles), as well as the corresponding signals after 0 h (open square) and 3 h (solid circle) under agitation in 1 M NaCl at 37°C. (C) Fluorescence spectra of 55 μM ANS in the presence of 0.02 mg mL−1 PDZ2, in 20 mM sodium phosphate, pH 7.0, 25°C (solid line), 20 mM sodium phosphate, 1 M NaCl, pH 7.0, 25°C (dashed line), and in 20 mM sodium phosphate, 1 M NaCl, pH 7.0, after 3 h agitation at 37°C (dotted line). The spectrum of 55 μM ANS in the absence of protein is also shown as a control (line with solid circles). (D) Urea-induced unfolding curves at equilibrium for 0.015 mg mL−1 PDZ2 in 20 mM sodium phosphate, pH 7.0, 25°C in the absence (open circles) and presence (solid circles) of 1 M NaCl. The reported curves were acquired using intrinsic fluorescence at 322 nm and normalized to the fraction of folded protein.