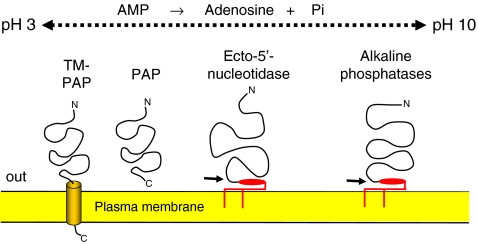
Fig. 1.
Membrane topology of enzymes capable of hydrolyzing extracellular AMP to adenosine. The splice variant (TM-PAP) of the soluble and secreted enzyme PAP is a type I transmembrane protein whereas ecto-5′-nucleotidase and the alkaline phosphatases are glycosylphosphatidyl inositol (GPI)-anchored. The anchors of both proteins can be cleaved (arrows) resulting in the formation of soluble enzymes. The pH optima of the three enzymes differ, PAP is very active at acid pH, ecto-5′-nucleotidase around neutral pH and alkaline phosphatases at highly alkaline pH. Whereas ecto-5′-nucleotidase selectively hydrolyzes nucleoside 5′-monophosphates, prostatic acid phosphatase and alkaline phosphatases hydrolyze a large variety of additional phosphomonoesters