Fig. 2.
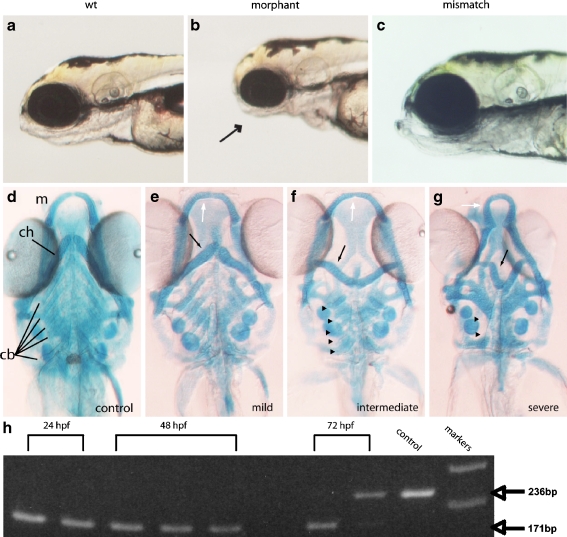
Loss of p2rx3.1 signaling results in defective pharyngeal skeleton formation by 5 dpf. Panel a shows a brightfield micrograph of a wild-type embryo, panel b is a morphant larva injected with MO-start, and panel c shows a larva injected with a control (mismatch) MO. The arrow points to the underdeveloped pharyngeal region in the morphant. Panel d shows a 5-dpf Alcian-Blue-stained control animal. Panels e–g show 5-dpf morphant larvae with differing degrees of cartilage deformations; e is a morphant larva with mild defects seen in the mandible (white arrow) and ceratohyal (black arrow) but with normal ceratobranchials, whereas f shows a morphant with more severe defects in the mandibular (white arrow) and ceratohyal (black arrow) cartilages but little disruption of ceratobranchials (arrowheads). However, in g, a morphant larvae shows severe affects on all pharyngeal cartilages- mandible (white arrow), ceratohyal (black arrow) as well as the near-total loss of ceratobranchial cartilages (arrowheads; m mandible, ch ceratohyal, cb ceratobrachials). PCR-mediated assessment of splice-morpholino efficacy is shown in panel h. cDNA was prepared from individual embryos at either 24, 48, or 72 hpf. Bands are PCR products generated from reactions containing either wild-type or MO-e10i10 morphant embryo-derived cDNAs using oligonucleotide primers that target sequences in exons 9 and 11. The distance spanning the primers in a wild-type cDNA containing exon10 is 236 bp, whereas a cDNA lacking exon 10 is 171 bp. By 72 hpf, the MO effects are being lost, most probably due to intracellular dilution brought about through multiple cell divisions, and properly spliced mRNA can be detected (upper band in second 72-hpf lane). Markers are 100-bp ladders corresponding to 200 and 300 bp