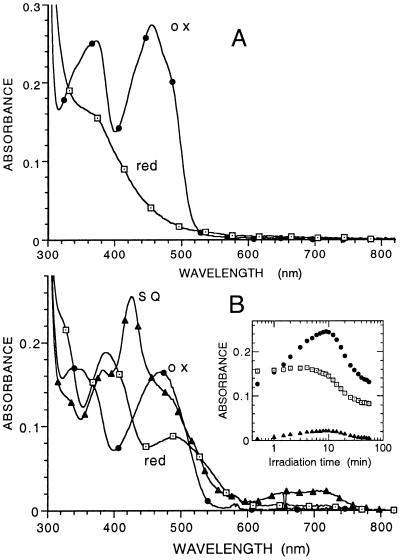
Figure 3.
Spectral properties of the fresh and aged forms of R268K. (A) Enzyme freshly purified. The spectra of the oxidized enzyme and that of the reduced enzyme after reduction by lactate are shown. (B) Enzyme after storage in ice for 3.5 mo in 10 mM imidazole/100 mM KCl buffer, pH 7.0. The spectrum identified with ● is that of the oxidized enzyme. The spectrum identified with ▴ is that formed maximally on irradiation with visible light under anaerobic conditions in the presence of 10 mM glycine and 1 μM 5-deazaflavin (11). The spectrum identified with □ is that of the fully reduced enzyme obtained after prolonged irradiation. The Inset shows the time course of the spectral changes at 425, 470, and 700 nm as a function of irradiation time. The spectrum of enzymed reduced by l-lactate was the same as that of the photochemically reduced form.