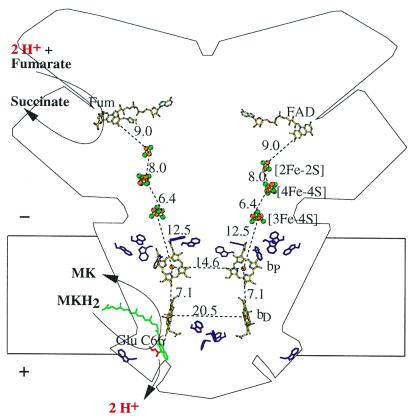
Figure 4.
Transmembrane electrochemical potential generation by W. succinogenes QFR coupling the two-electron oxidation of menaquinol (MKH2) to menaquinone (MK) to the two-electron reduction of fumarate to succinate. The positive (+) and negative (−) sides of the membrane are indicated. The prosthetic groups of the QFR dimer are displayed (coordinate set PDB entry 1QLA; ref. 5). Distances between prosthetic groups are edge-to-edge distances in Å as defined in ref. 37. Distances shorter than 14 Å (i.e., within one QFR monomer, but not between the two monomers of the dimer) are considered to be relevant for physiological electron transfer. Also drawn are the side chains of Glu-C66 (in red) and of the subunit C Trp residues (purple). The latter are markers for the hydrophobic surface-to-polar transition zone of the membrane. The position of bound fumarate (Fum) is taken from PDB entry 1QLB (5). The tentative model of menaquinol binding (drawn in green) is taken from Fig. 1. Its edge-to-edge distance to heme bD is 6.7 Å.