Abstract
Aims
Renal dysfunction is an important independent prognostic factor in heart failure (HF). Cardiac resynchronization therapy (CRT) improves functional status and left ventricular (LV) function in HF patients with ventricular dyssynchrony but the impact of CRT on renal function is less defined. We hypothesized that CRT would improve glomerular filtration rate as estimated by the abbreviated MDRD equation (eGFR).
Methods and Results
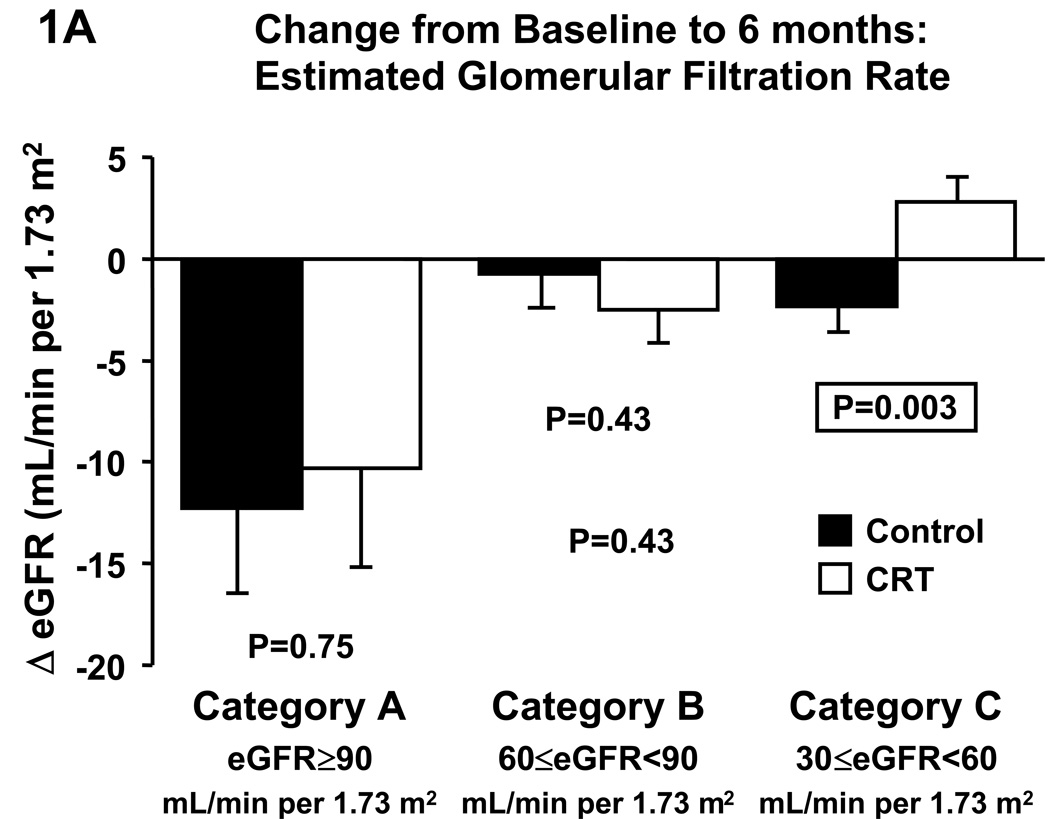
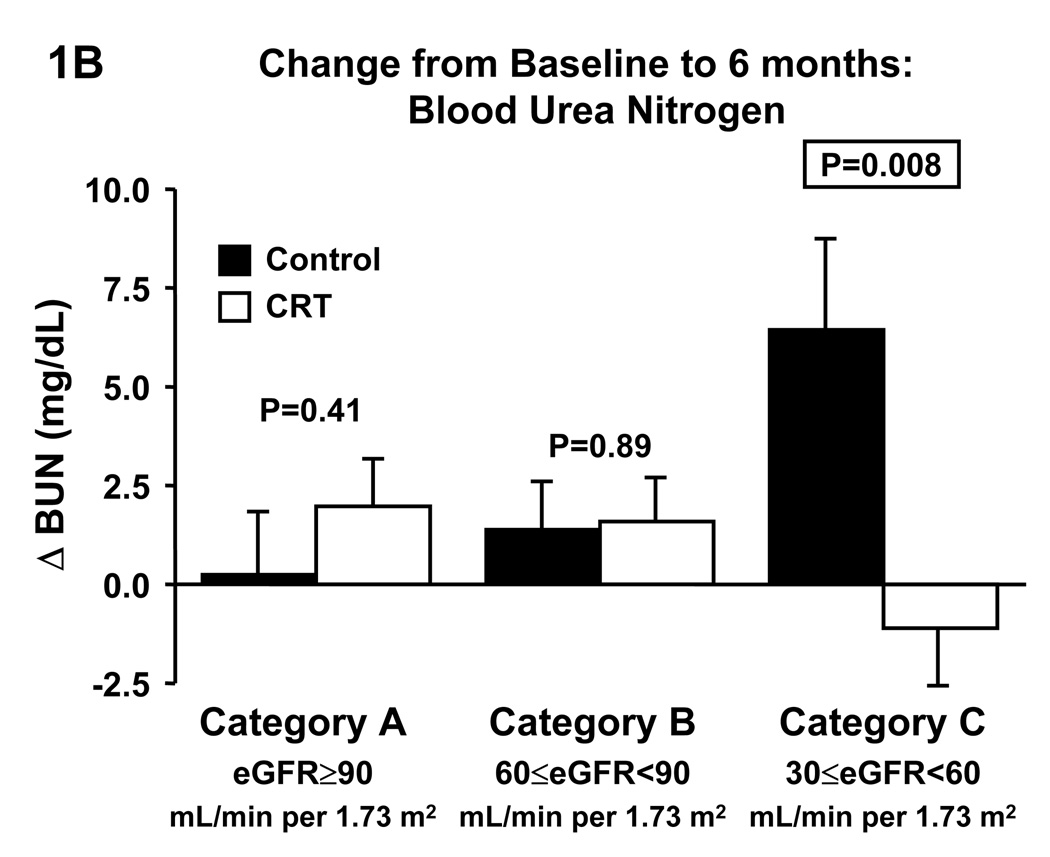
The Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study evaluated CRT in HF patients with NYHA class III–IV, ejection fraction ≤35%, and QRS ≥130 ms. Patients were evaluated before and 6 months after randomization to control (n=225) or CRT (n=228). Patients were categorized according to their baseline eGFR: ≥90 (category A), 60≤eGFR<90 (category B), and 30≤eGFR<60 (category C) mL/min per 1.73 m2. CRT improved LV function in all categories. Compared to control, CRT increased eGFR (−2.4±1.2 vs. +2.7±1.2 mL/min per 1.73 m2; p=0.003) and reduced blood urea nitrogen (+6.4±2.4 vs. −1.1±1.5 mg/mL; p=0.008) in category C, whereas no differences were observed in categories A and B.
Conclusion
CRT increased eGFR and reduced blood urea nitrogen in HF patients with moderately reduced baseline eGFR. By improving cardiac function, CRT can indirectly improve renal function, underscoring the importance of cardiorenal interaction and providing another mechanism for the beneficial effects of CRT.
Keywords: Heart failure, renal dysfunction, cardiac resynchronization therapy, glomerular filtration rate, neurohormones, natriuretic peptides
INTRODUCTION
Renal function as assessed by glomerular filtration rate (GFR) has emerged as a powerful independent prognostic factor in patients with heart failure (HF) underscoring the link between the heart and the kidney.1–6 Renal function can be impaired due to intrinsic renal disease and secondarily to cardiac dysfunction. In the latter case, improving cardiac function should improve renal function and possibly contribute to improved outcome.
Cardiac resynchronization therapy (CRT) has been demonstrated to be an efficacious therapy for patients with reduced left ventricular (LV) dysfunction and ventricular dyssynchrony, improving LV function, symptomatic and functional status, and survival.7–11 However, little is known about the effect of CRT on renal function.12,13 To the best of our knowledge, no data from placebo-controlled trials has been reported to date.
The MIRACLE trial was a large, double-blind, randomized, and placebo-controlled multicenter trial to assess the efficacy of CRT in HF patients with LV systolic dysfunction and intraventricular conduction delay. Patients underwent comprehensive characterization of their functional capacity, LV function, neurohormones, and standard blood chemistry. CRT resulted in significant improvements in functional status and LV function as assessed by echocardiograpy.8,9
In the current study we hypothesized that CRT would also improve renal function. We therefore assessed the effect of CRT on estimated GFR (eGFR). Specifically, we assessed the eGFR response in those with normal and impaired eGFR as calculated by the abbreviated Modification of Diet in Renal Disease (MDRD) equation.
METHODS
The present study is a retrospective analysis of the MIRACLE trial, which has been reported in detail previously.8,9 Only patients with paired eGFR values at baseline and at 6 months were included in this analysis.
Study Population
Enrollment criteria for the MIRACLE trial were: New York Heart Association (NYHA) symptom class III or IV HF, QRS duration ≥130 ms, LV ejection fraction ≤35%, and LV end diastolic diameter ≥55 mm.8 In addition, patients had to be on a stable medical regimen for at least a month (beta-blockers: three months). An exclusion criterion was serum creatinine>3.0 mg/dL. The institutional review board at each center approved the study protocol, and written informed consent was obtained from all patients.
MIRACLE Study Design
After enrollment, patient evaluation at baseline included assessment of clinical status (NYHA class, 6-minute hall walk, Minnesota Living with Heart Failure questionnaire, cardiopulmonary exercise testing with the modified Naughton protocol), electrocardiogram, and echocardiography.8,9,14 Plasma samples for neurohormonal measurements were sent frozen (−20°C or below) to the neurohormonal core laboratory (Mayo Clinic), and stored at −80°C until analysis. Within two weeks of the baseline blood draw the resynchronization device was implanted. Patients were randomized to a control group (resynchronization device OFF, n=225) and CRT (resynchronization device ON, n=228) and reevaluated after 6 months.
Blood chemistry
Serum creatinine, blood urea nitrogen (BUN), hemoglobin, and hematocrit were determined at the individual participating sites. Given that follow-up was done at the same institution, individual patient data should accurately reflect changes over time despite the probable lack of standardization between laboratories.
Estimated GFR Categories
For this study, patients were categorized according to their baseline eGFR. GFR was estimated with the abbreviated Modification of Diet in Renal disease (MDRD) equation: eGFR (in mL/min per 1.73 m2) = 186.3 × serum creatinine−1.154 × age−0.203 × (0.742 if female) × (1.21 if black).15,16 Cutoffs for eGFR categories were based upon those used by the National Kidney Foundation for the classification of chronic kidney disease.17 Category A was defined as normal or increased eGFR (≥90 mL/min per 1.73 m2), category B as mildly reduced eGFR (60≤eGFR<90 mL/min per 1.73 m2), and category C as moderately reduced eGFR (30≤eGFR<60 mL/min per 1.73 m2). There were only 12 control and 9 CRT patients with an eGFR<30 mL/min 1.73 m2 (category D). As this category was too small for a meaningful statistical analysis, these patients were not considered in the current study.
Worsening Renal Function
Patients were defined as having “worsening renal function” if they fulfilled at least one of the following three criteria: increase in serum creatinine by at least 0.3 mg/dL, decrease in eGFR by at least 25%, or increase in BUN by at least 25%, between baseline and 6 months.
Assays
Neurohormones analyzed included atrial natriuretic peptide (ANP, Phoenix Pharmaceuticals, Mountain View, CA, USA),18 B-type natriuretic peptide (BNP, Shionogi),19 norepinephrine, plasma renin activity, aldosterone, and Big endothelin. Missing data were due to samples arriving thawed at the core laboratory or sample volume being too low to run all assays.
Statistics
Control and CRT were compared with unpaired Student’s t-test for normally distributed continuous variables, Mann-Whitney U-test for not normally distributed continuous variables, and chi square test or Fisher’s exact test for dichotomous variables. Estimated GFR categories were compared with one-way analysis of variance and post-hoc Bonferroni test or Kruskal-Wallis test and post-hoc Mann-Whitney U-test with Sidak adjustment. The effect of CRT on eGFR and other parameters was assessed by comparing changes from baseline to 6 months between the control and CRT groups by unpaired t-test or Mann-Whitney U-test in the respective eGFR categories. ANP and BNP were analyzed with log-transformed data and parametric tests. A two-sided p≤0.05 was considered to indicate statistical significance. Analyses were performed with SPSS for Windows Version 13.0 (Chicago, IL).
RESULTS
Baseline characteristics of the 453 patients participating in the MIRACLE trial were reported previously with no significant differences between control and CRT groups.8 However, patients randomized to CRT in eGFR category C were more likely to be on a beta blocker at baseline (control: 44% vs. CRT 63%, p=0.02). Of the 448 patients which had baseline creatinine values available, 16% had a normal eGFR (category A), 40% had mildly impaired renal function (category B), and 39% had moderately decreased eGFR (category C). Five percent had severely decreased eGFR (category D) and were excluded from the analyses. Twenty-seven patients died (category A: control 3, CRT 2; category B: control 5, CRT 6; category C: control 6, CRT 3; category D: control 2, CRT 0). Two control patients underwent heart transplantation (one each in category B and C) and one control and one CRT patient stopped participation due to a pacemaker-related infection. For 13 patients no follow-up creatinine was available and one patient whose 6-month serum creatinine was reported as 0.06 mg/dL was excluded, leaving 384 patients for the current analyses.
Baseline characteristics
Baseline characteristics for eGFR categories A–D combined and separately for eGFR categories A, B, and C are shown in Table 1. There were no significant differences between control and CRT groups when categories A–D were combined (data not shown). Patients with lower eGFR were older, more likely to have an ischemic HF etiology, had a lower body mass index and hemoglobin level, were more likely to be on a diuretic, and tended to be less likely to be on a beta blocker. The use of nitrates, digoxin, and calcium channel blockers was similar (data not shown). As expected, serum creatinine and blood urea nitrogen increased significantly with decreasing eGFR. Mean arterial pressure was lower in category C and more people were in NYHA class IV, whereas 6-minute hallwalk distance was similar across categories. Estimated GFR categories did not differ in LV ejection fraction, LV volumes, or cardiac index, although LV ejection fraction tended to be lower in eGFR category A. However, mitral regurgitation area was significantly higher in category C as compared to A and B. Furthermore, ANP, BNP, norepinephrine, aldosterone, and Big endothelin were significantly higher and plasma renin activity tended to be so.
Table 1.
General baseline characteristics in groups defined by baseline eGFR.
| Characteristic | Cat. A–D | Category A eGFR≥90 mL/min per 1.73 m2 |
Category B 60≤eGFR<90 mL/min per 1.73 m2 |
Category C 30≤eGFR<60 mL/min per 1.73 m2 |
p-value |
|---|---|---|---|---|---|
| N | 403 | 67 | 160 | 155 | |
| eGFR, mL/min per 1.73 m2 | 65.5±1.2 | 104.5±2.1 | 72.9±0.6 | 46.2±0.7 | |
| CRT, % | 52.4 | 56.7 | 51.3 | 52.9 | 0.75 |
| Age, years | 64±1 | 55±1b,c | 62±1a,c | 68±1a,b | <0.001 |
| Male sex, % | 67.7 | 64.2 | 66.9 | 71.6 | 0.48 |
| Ischemic etiology, % | 53.8 | 37.3c | 48.8c | 65.2a,b | <0.001 |
| Body mass index, kg/m2 | 28.7±0.4 | 29.4±0.81 | 29.8±0.7c | 27.2±0.5b | 0.008 |
| Diabetes, % | 31.8 | 34.3 | 27.5 | 32.9 | 0.47 |
| Hemoglobin, g/dL | 13.1±0.1 | 13.4±0.2 | 13.4±0.1c | 12.9±0.1b | 0.006 |
| Serum creatinine, mg/dL | 1.26±0.02 | 0.79±0.02b,c | 1.03±0.01a,c | 1.56±0.02a,b | <0.001 |
| Blood urea nitrogen, mg/dL | 29.6±0.9 | 17.9±1.2b,c | 23.2±.1.0a,c | 38.2±1.5a,b | <0.001 |
| NYHA class III/IV, % | 92/8 | 97/3 | 95/5c | 87/14b | 0.005 |
| 6-minute hallwalk, m | 303±4 | 304±11 | 306±7 | 303±7 | 0.99 |
| Mean blood pressure, mmHg | 84±1 | 86±1c | 85±1 | 82±1a | 0.013 |
| LV ejection fraction, % | 24±1 | 22±1 | 25±1 | 24±1 | 0.09 |
| LV end diastolic volume, mL | 301±6 | 310±16 | 302±11 | 297±10 | 0.77 |
| LV end systolic volume, mL | 234±6 | 247±15 | 234±10 | 230±9 | 0.62 |
| Cardiac index, L/min per m2 (VTI) | 1.57±0.03 | 1.65±0.09 | 1.58±0.05 | 1.52±0.05 | 0.30 |
| MR jet area, cm2 | 7.47±0.34 | 5.0±0.6c | 6.3±0.4c | 9.2±0.6a,b | <0.001 |
| LV mass index, g/m2 | 182±3 | 181±6 | 180±5 | 182±4 | 0.97 |
| ANP, pg/mL | 86 (38/171) | 47 (28/98)c | 75 (31/137)c | 119 (48/201)a,b | <0.001 |
| BNP, pg/mL | 456 (141/1172) | 208 (56/490)c | 448 (130/1007)c | 661 (204/1633)a,b | <0.001 |
| PRA, ng·mL−1·h−1 | 10.9 (2.9/18.6) | 8.3 (2.8/14.1) | 10.0 (2.0/19.6) | 12.4 (4.1/19.1) | 0.08 |
| Aldosterone, ng/dL | 9.9 (5.5/18.7) | 7.4 (4.2/11.6)c | 10.0 (5.2/19.0) | 10.9 (6.3/20.4)a | 0.004 |
| Norepinephrine, pg/mL | 391 (261/587) | 359 (176/495)c | 344 (243/501)c | 456 (304/683)a,b | <0.001 |
| Big Endothelin, pg/mL | 9.9 (5.9/15.9) | 7.0 (4.4/12.7)c | 8.0 (5.8/12.1)c | 12.4 (7.7/22.1)a,b | <0.001 |
| Diuretic, % | 94 | 90c | 92 | 97a | 0.039 |
| ACEi/ ARB, % | 93 | 97 | 93 | 92 | 0.41 |
| Betablocker, % | 60 | 70 | 63 | 54 | 0.06 |
Values are mean±SEM, percentage, or median (25th/75th percentile). P-value is for comparison of Categories A–C.
p<0.05 vs. group A
p<0.05 vs. group B
p<0.05 vs. group C.
ANP, atrial natriuretic , BNP, B-type natriuretic peptide, ACEi/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, CRT, cardiac resynchronization therapy, eGFR, estimated glomerular filtration rate. LV, left ventricular, MR mitral regurgitation, NYNA New York Heart Association, PRA, plasma renin activity..
Changes from Baseline to 6-month Follow-up in eGFR Categories
With all eGFR categories combined (A–D), eGFR decreased in the control group by −2.9±1.1 mL/min per 1.73 m2 and in the CRT group by −1.5±1.1 mL/min (p=0.37 between groups), while BUN increased by 2.7±1.1 mg/dL in the control group and 0.6±0.8 mg/dL in the CRT group (p=0.11 between groups). Changes from baseline to follow-up at 6 months in eGFR categories for the control and CRT groups, respectively, are shown in Figure 1, and Table 2.
Figure 1.
Effect of cardiac resynchronization therapy on estimated glomerular filtration rate (A) and blood urea nitrogen (B) in categories of baseline estimated glomerular filtration rate. BUN, blood urea nitrogen, CRT, cardiac resynchronization therapy, eGFR, estimated glomerular filtration rate.
Table 2.
Mean changes of functional parameters from baseline to 6 months in categories of baseline eGFR.
| Category A eGFR≥90 mL/min per 1.73 m2 |
p | Category B 60≤eGFR<90 mL/min per 1.73 m2 |
p | Category C 30≤eGFR<60 mL/min per 1.73 m2 |
p | ||
|---|---|---|---|---|---|---|---|
| N | Control | 29 | 78 | 73 | |||
| CRT | 38 | 82 | 82 | ||||
| NYHA class | Control | −0.41±0.15 (29) | 0.006 | −0.39±0.07 (75) | <0.001 | −0.44±0.08 (71) | <0.001 |
| CRT | −0.86±0.10 (37) | −0.83±0.09 (81) | −0.89±0.08 (80) | ||||
| 6-minute hallwalk, | Control | +33±21 (29) | 0.16 | +17±11 (76) | 0.12 | −6±12 (71) | 0.01 |
| m | CRT | +40±18 (38) | +37±9 (82) | +13±12 (81) | |||
| Mean blood pressure, | Control | +1.0±2.5 | 0.06 | +1.1±1.5 | 0.43 | −0.7±1.3 | 0.02 |
| mmHg | CRT | −4.5±1.8 | +2.7±1.5 | +3.9±1.4 | |||
| LV ejection | Control | +2.3±1.5 (25) | 0.016 | 0.0±0.9 (55) | <0.001 | 0.0±0.8 (51) | 0.001 |
| fraction, % | CRT | +7.0±1.2 (26) | +5.7±1.1 (62) | +4.4±0.9 (60) | |||
| LV end diastolic | Control | −8±11 (25) | 0.010 | +15±7 (55) | <0.001 | +11±9 (51) | 0.003 |
| volume, mL | CRT | −52±12 (26) | −38±7 (62) | −30±10 (60) | |||
| LV end systolic | Control | −12±12 (25) | 0.012 | +13±6 (55) | <0.001 | +8±8 (51) | 0.002 |
| volume, mL | CRT | −53±10 (26) | −40±7 (62) | −30±9 (60) | |||
| Cardiac index, | Control | −0.16±0.14 (18) | 0.45 | −0.08±0.09 (40) | 0.074 | 0.00±0.09 (42) | 0.54 |
| L/min per m2 (VTI) | CRT | 0.00±0.14 (24) | 0.14±0.08 (49) | 0.07±0.07 (53) | |||
| Mitral regurgitation | Control | +0.6±1.5 (21) | 0.045 | −0.5±0.5 (47) | 0.003 | −0.7±0.8 (41) | 0.009 |
| jet area, cm2 | CRT | −2.7±0.6 (22) | −3.1±0.7 (45) | −3.7±0.7 (43) | |||
| LV mass, | Control | −11±10 (19) | 0.90 | +14±10 (39) | 0.162 | +19±9 (39) | 0.006 |
| g | CRT | −8±16 (22) | −6±10 (51) | −23±11 (44) | |||
| Serum creatinine | Control | +0.09±0.03 (29) | 0.48 | +0.05±0.03 (78) | 0.59 | +0.16±0.05 (73) | 0.001 |
| mg/dL | CRT | +0.13±0.04 (38) | +0.07±0.03 (82) | −0.04±0.03 (82) | |||
| Atrial natriuretic | Control | +8 (−34/85) | 0.54 | −3 (−69/61) | 0.67 | −2 (−77/63) | 0.02 |
| peptide, pg/mL | CRT | +6 (−30/23) | −8 (−50/29) | −14 (−88/22) | |||
| B-type natriuretic | Control | 0 (−385/238) | 0.45 | −9 (−383/225) | 0.16 | −268 (−1035/52) | 0.61 |
| peptide, pg/mL | CRT | −33 (−176/34) | −166 (−475/86) | −125 (−792/193) |
Values are mean±SEM or median (25th/75th percentile. P-values are Control vs. CRT in the respective category. eGFR, estimated glomerular filtration rate, LV, left ventricular, NYHA, New York Heart Association.
Change in Renal Function in eGFR Categories
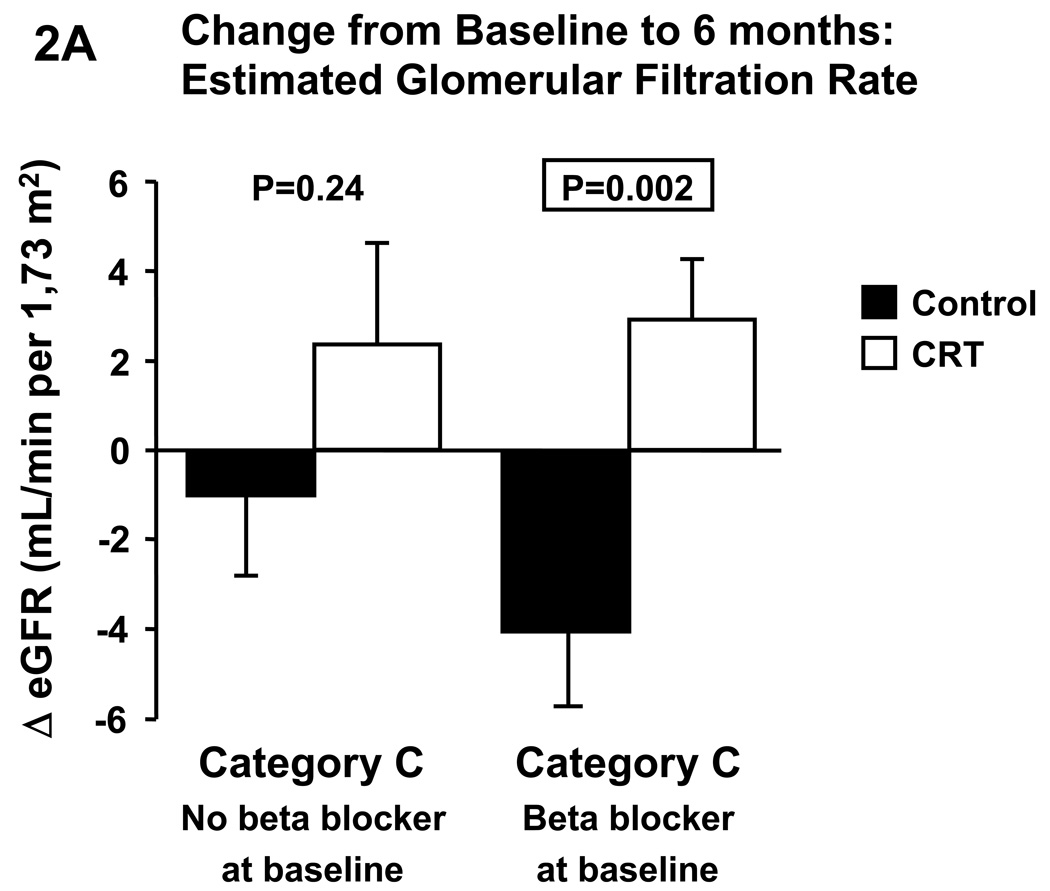
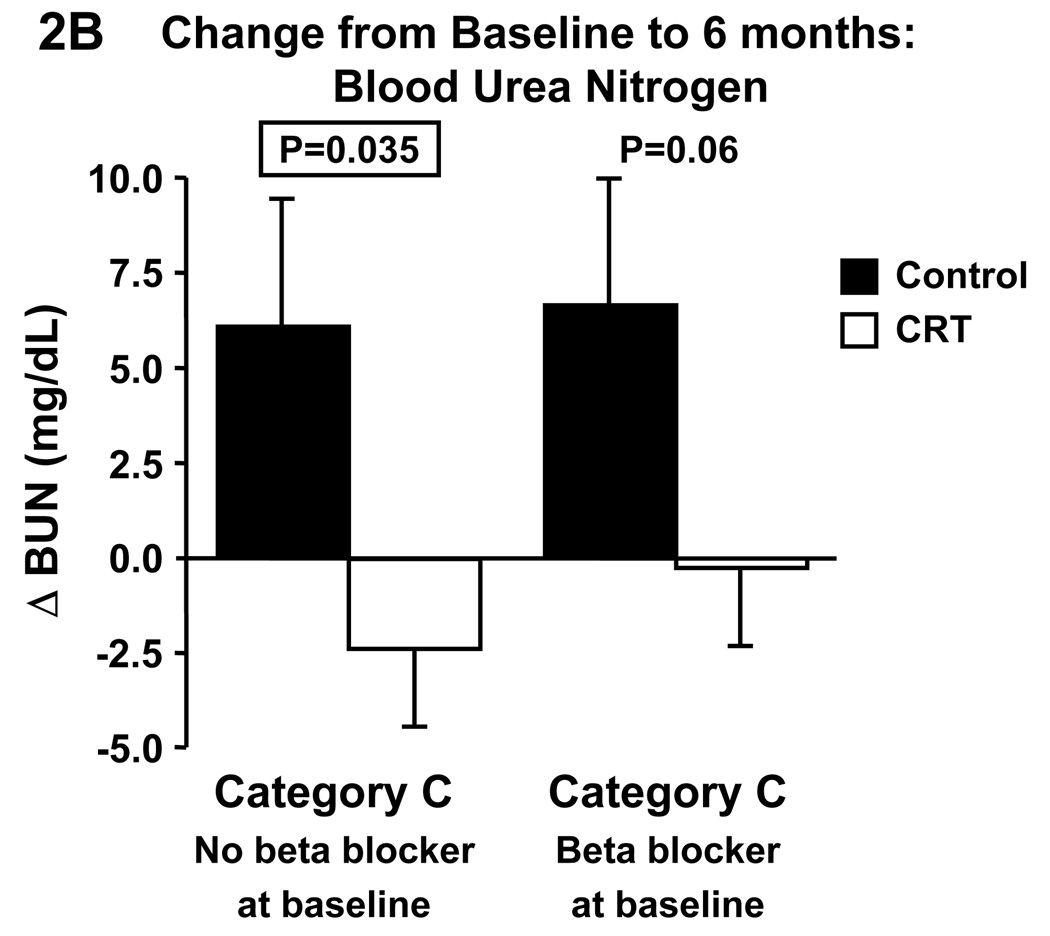
Changes in eGFR and blood urea nitrogen were not different between CRT and control in category A or B. In contrast, CRT significantly increased eGFR (Fig. 1A) and decreased blood urea nitrogen (Fig. 1B) compared to control in category C. Given that CRT patients in category C were more likely to be on a beta blocker we repeated the analyses in this category with patients divided according to beta blocker medication at baseline (Fig.2). In patients without beta blocker at baseline, CRT did not affect changes in eGFR, whereas serum creatinine tended to decrease (p=0.07) and BUN significantly decreased compared to control. In patients on beta blocker at baseline, CRT significantly increased eGFR and decreased serum creatinine (p=0.003), and BUN tended to decrease (p=0.06).
Figure 2.
Effect of cardiac resynchronization therapy on estimated glomerular filtration rate (A) and blood urea nitrogen (B) in category C patients (i.e. 30≤eGFR<60 mL/min/1.73m2) with and without beta blocker medication at baseline. BUN, blood urea nitrogen, CRT, cardiac resynchronization therapy, eGFR, estimated glomerular filtration rate.
Worsening Renal Function
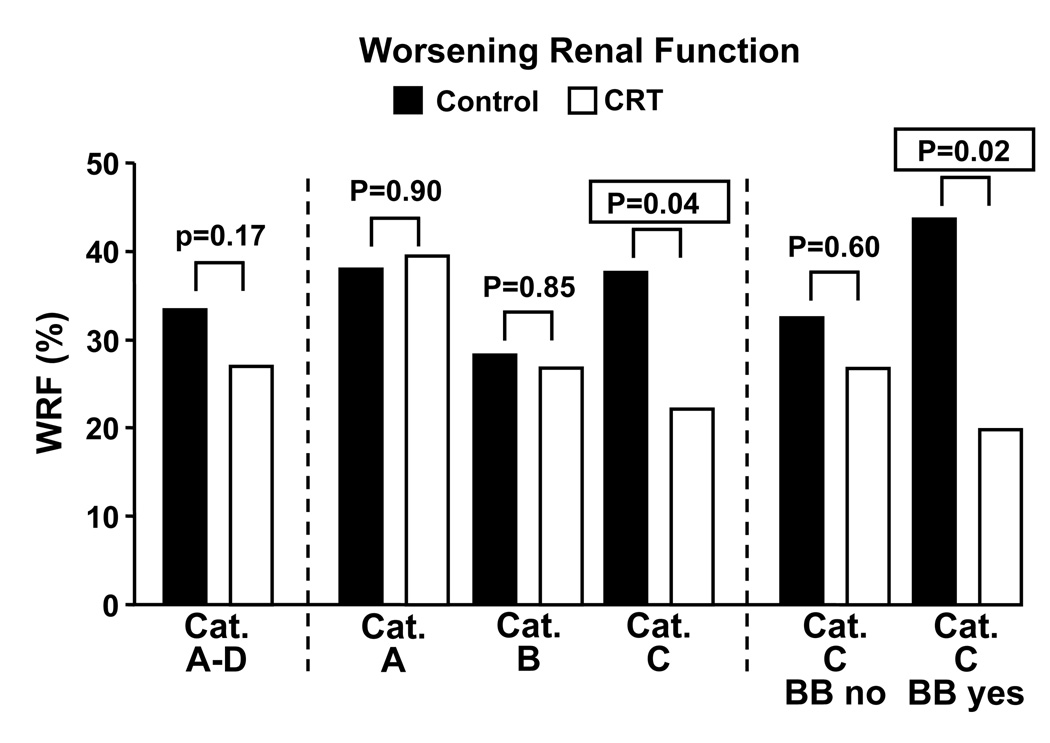
The percentage of patients that fulfilled the definition of worsening renal function is shown in Fig.3A. There was no difference in the percentage in eGFR categories A and B, while there was a significant reduction in category C. Figure 3 also reports that the decreased incidence of worsening renal function in category C occurs in patients with beta blocker use at baseline, whereas there was no difference in patients without beta blocker use at baseline.
Figure 3.
Effect of cardiac resynchronization therapy on percentage of patients which fulfill the definition of “worsening renal function” in different categories of baseline eGFR. Category C is further divided into patients with and without and beta blocker medication at baseline. BB, beta blocker medication at baseline, BUN, blood urea nitrogen, CRT, cardiac resynchronization therapy, eGFR, estimated glomerular filtration rate, WRF, worsening renal function.
Change in Functional Capacity and Cardiovascular Parameters
CRT improved NYHA class compared to control in all three eGFR categories. Six-minute hallwalk distance significantly improved with CRT in category C with a trend in categories A and B. Mean arterial pressure tended to decrease with CRT in category A but increased significantly in category C. In all three eGFR categories, CRT significantly increased LV ejection fraction and decreased LV volumes as compared to control. Cardiac index estimated by product of velocity time integral and cross-sectional area of LV outflow tract tended to increase in category B. Mitral regurgitation jet area decreased in all three eGFR categories. Changes in LV mass were not different in category A and B, whereas CRT significantly decreased LV mass in category C.
Changes in Blood Chemistry and Neurohumoral Activation
There were no differences in changes of hemoglobin. CRT decreased serum creatinine and ANP as compared to the control group in eGFR category C. In contrast, changes in BNP, plasma renin activity, aldosterone, norepinephrine, and Big endothelin were similar between randomization groups in all eGFR categories (data not shown).
Change in Medication
No significant differences in discontinuing or introducing drugs were observed between CRT and control groups (data not shown). In both control and CRT groups, 86% had no introduction or discontinuation of beta blockers, diuretics, ACE inhibitors, nitrates, or calcium channel blockers as compared to baseline. Diuretics were introduced in 2.1% and discontinued in 2.6% of control patients as compared to 1.9% and 4.3%, respectively, in the CRT group. We did not assess dose titration.
DISCUSSION
This study reports for the first time that CRT in a randomized placebo-controlled clinical trial in human HF improves eGFR and reduces blood urea nitrogen in patients with moderately decreased eGFR at baseline (30–59 mL/min per 1.73 m2). We also found that decreased baseline eGFR is associated with increased age, ischemic HF etiology, more severe mitral regurgitation, reduced blood pressure, and increased neurohumoral activation.
While there is a large body of evidence implicating a decline in eGFR and increased blood urea nitrogen with increased HF mortality,1,20 little is known about the effect of CRT on renal function. Fung et al. reported that after three months of CRT, patients with reverse remodeling (defined as at least 10% reduction in LV end systolic volume), had a mean increase of eGFR from an average of 51.7 to 54.2 mL/min per 1.73 m2, while patients without reverse remodeling had a decrease from a mean 61.9 to 48.8 mL/min per 1.73 m2.13 The major limitation of this study was the lack of a control group. Cowburn et al. reported that in ten patients with severe decompensated HF on inotropic support, CRT enabled successful weaning from inotropes and reduced serum creatinine.12 This study also did not include a control group, the patients had more severe HF than most patients included in the large CRT clinical trials, and due to the discontinuation of the inotropes there was a significant change in medication. To the best of our knowledge, our study is the first large placebo-controlled trial to report that CRT can improve estimated eGFR and reduce blood urea nitrogen as compared to control in patients with impaired renal function.
As in other clinical trials in HF, there was a range of eGFR at baseline. This provided us with the opportunity to better characterize factors associated with impaired eGFR advancing insights into the phenotype of the patient with stage C/D heart failure with reduced GFR. We observed that patients with lower eGFR were older, weighed less, were more likely to have an ischemic HF etiology, and had lower mean and diastolic blood pressures and therefore reduced renal perfusion pressure. They also were more likely to be in NYHA class IV at baseline and had lower peak oxygen consumption. Interestingly, 6-minute hallwalk distance and prevalence of diabetes were similar. Patients in the lowest eGFR category were more likely to use diuretics and tended to be less likely to use beta blockers. Hemoglobin levels were lower and serum creatinine and blood urea nitrogen higher. While LV volumes and cardiac index were similar among categories, mitral regurgitation jet area was significantly higher in patients with the lowest eGFR. The increased mitral regurgitation may also be associated with higher right-sided pressures and increased pressure in the inferior vena cava, thus impairing kidney function by decreasing renal perfusion pressure.21 Reduced eGFR was also associated with a neurohumoral profile consistent with increased cardiac stretch due to increased filling pressure (increased ANP, BNP), decreased renal perfusion (increased plasma renin activity, aldosterone), and increased activation of the sympathetic nervous system (increased norepinephrine). Big endothelin, the prohormone of the potent vasoconstrictor endothelin, has previously been reported to increase with the severity of HF.22 These neurohumoral findings are consistent with previous reports.3,23 Thus, our findings are consistent with the concept that impaired cardiovascular hemodynamics and neurohumoral activation may play a seminal role in the impaired renal function which characterizes severe HF.
At follow-up, eGFR changes were not different between control and CRT groups in patients with normal (category A) and mildly reduced (category B) eGFR. However, CRT significantly improved eGFR compared to control in patients with moderately decreased eGFR (category C, i.e. 60>eGFR≥30 mL/min). This was associated with a significant reduction in blood urea nitrogen, which has been identified as an important prognostic factor in patients admitted for HF.20 Due to baseline differences in beta blocker therapy we also analyzed changes in category C separately for patients with and without beta blocker medication at baseline. Significant results for an improvement in eGFR and creatinine were observed in the subgroup with beta blocker. In keeping with these results is the reduced percentage of patients who fulfilled the criteria for “worsening renal function” in CRT patients in category C with beta blocker therapy. While we cannot exclude a specific interaction between CRT and beta blocker therapy to improve renal function, the most likely explanation for the lesser degree of improvement in patients without beta blocker is the disease condition that precluded them from receiving a beta blocker in the first place. Indeed, subgroup analyses of the COMPANION and CARE-HF trial showed that the respective primary endpoints were significantly improved with CRT only in patients with beta blocker.
The lack of eGFR change as compared to the control group in eGFR categories A and B may be due to the fact that no improvement would be required nor expected if baseline eGFR were normal and that an improvement with only mildly reduced eGFR may be difficult to detect. It should be noted that the apparently large decrease in eGFR in category A (average reduction in both control and CRT ≥10 mL/min per 1.73 m2) is due to an increase in serum creatinine smaller than that observed in the control group in category C, which has an average eGFR reduction of 2.3 mL/min per 1.73 m2. It should also be noted that there was on average an increase in serum creatinine in the control groups across the eGFR categories. This may simply reflect the natural history of HF, but renal function may also have declined secondary to the contrast medium administered during the device implantation.24 It should also be noted that part of the observed changes may be attributable to the “regression to the mean” phenomenon. However, this regression would be expected to occur to a similar degree in both randomization groups so that it cannot explain the improved renal parameters in category C with CRT in this study. Based on our observations it would be worthwhile to analyze in larger CRT trials that were designed to evaluate hospitalization and mortality whether changes in eGFR affected outcome.
Unlike many other interventions such as drug therapy, CRT does not have direct effects on the kidney. Renal function may however be affected indirectly by a variety of factors. CRT has been reported to improve symptoms, functional status, LV function, as well as morbidity and mortality.7–11 The primary mechanism by which this is achieved is improved ventricular pumping efficiency through better temporal coordination of left ventricular activation and thus contraction, which may result in increased LV pressure generation, mean arterial pressure, cardiac output, stroke work, myocardial efficiency, and reduced mitral regurgitation and cardiac filling pressures.9–11,25–29 Indeed, CRT significantly increased mean arterial pressure in category C, while it reduced mitral regurgitation in all eGFR categories. Increasing aortic pressure and decreasing right-sided pressures would increase renal perfusion pressure and thus improve eGFR. Further, ANP, which is primarily secreted in response to myocardial stretch, was reduced with CRT in category C, which would be consistent with reduced cardiac filling and consequently renal vein pressure thus contributing to improved eGFR. St John Sutton et al. reported that cardiac index estimated using the velocity time integral increased compared to control in the MIRACLE trial.9 This increase in cardiac index with CRT was not apparent when it was estimated by the change in LV volume, which can be explained by the simultaneous reduction in mitral regurgitation and thus relatively smaller required stroke volume.
Besides these hemodynamic factors, another possible mechanism for enhanced renal function could be a decreased activation of the sympathetic nervous system, which has been reported for CRT. Plasma norepinephrine, a rough estimate of sympathetic activity with important limitations,30 was not reduced with CRT in this study, which is similar to what has been reported in other studies.31–33 While Adamson et al. reported that they also did not observe a change in plasma catecholamines, heart rate variability, which is influenced by both the sympathetic and parasympathetic system, was significantly improved with CRT.34 Furthermore, Grassi et al reported that CRT reduced muscle sympathetic nerve traffic.32 Other neurohumoral factors may also play a role. Increased blood pressure would be expected to decrease activation of the renin-angiotensin-aldosterone system and possibly the endothelin system as well. However, we saw no significant differences between CRT and control in these neurohormones. Of note, in univariate analysis with categories A–D combined change in eGFR correlated weakly but significantly with changes in aldosterone (rSpearman= −0.24 (category C only: rSpearman= −0.39)), mean blood pressure (rSpearman= +0.23), plasma renin activity (rSpearman= −0.20), and norepinephrine (rSpearman= −0.15). These correlations are in keeping with reduced neurohumoral activation due to increased systemic and renal perfusion pressure. Interestingly, if patients were classified as responders or non-responders using a variety of criteria (relative increase of LVEF≥25%, relative reduction of LVESV>15%, improvement of ≥1 NYHA class) we found no significant differences in eGFR between responders and non-responders in any of the eGFR categories (or all categories combined) with any of the definitions (data not shown). This lack of association may be due to a lack of statistical power or due to the complexity of the cardiorenal interaction. Indeed, renal function may improve through the cumulative effects of relatively small changes in e.g. increased perfusion pressure, reduced venous congestion, and decreased neurohumoral activation.
This study provides several other observations that can be considered hypothesis-generating for future studies. In all three eGFR categories CRT improved LV ejection fraction, volumes, and mitral regurgitation jet area. However, LV mass only decreased in category C (p=0.02 for patients without beta blocker, p=0.10 for patients with beta blocker) with a trend in category B. The mechanism and importance of the reduction in LV mass warrants further study. In addition, CRT in patients in category C significantly improved NYHA class and 6–minute hallwalk. While category C due to its baseline characteristics (greatest impairment of baseline eGFR, age, ischemic etiology) can be expected to generally have a poorer prognosis, these observations suggest that these patients still derive benefit as compared to their controls. While we do not report hospitalization and mortality in this study, Cleland et al in the CARE-HF trial reported that CRT significantly reduced the composite primary endpoint of all cause mortality or an unplanned hospitalization for a major cardiovascular event in patients both above and below the median eGFR of the study population, which was 60.3 mL/min per 1.73 m2.11 Furthermore, a pre-defined analysis of the CARE-HF trial showed that while baseline eGFR was a significant predictor of outcome, it did not affect the subsequent benefit from CRT, unlike baseline systolic blood pressure and interventricular mechanical delay.35
Our study has all the limitations of a retrospective analysis of prospectively collected data. The MIRACLE investigators were not blinded to the serum creatinine, and may have made treatment decisions during the care of study subjects based on the serum creatinine such as introducing, discontinuing, or titrating medications, which are actions for which we did not account in our analyses. Further, while patients with a baseline serum creatinine >3 mg/dL were excluded from the MIRACLE trial, it is likely that a substantial number of patients would have some intrinsic renal disease; this however, would make it less likely to demonstrate an improvement with CRT. We also recognize that a recent study in HF patients found that the abbreviated MDRD equation as used in this study overestimated GFR in the lower and underestimated GFR in the higher ranges of GFR measured by 125I-iothalamate clearance.36 Thus, our findings may apply to those patients who have a true GFR somewhat above 60 mL/min per 1.73 m2. Finally, we do not have a large enough sample size to extend our inferences to long-term mortality; however, based on the available data, we would surmise that those patients who had an improvement in eGFR would be likely to have improved mortality over the long-term.
In summary, the findings of this study suggest that CRT in addition to its known beneficial effects regarding LV function and remodeling and symptomatic and functional status also may improve renal function. Similar subgroup analyses of some of the more recent clinical trials, which were larger, had longer follow-up, and more contemporary drug therapy, may help to clarify whether CRT can be considered a renal protecting and enhancing therapeutic strategy in HF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 2.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 4.Mahon NG, Blackstone EH, Francis GS, Starling RC, 3rd, Young JB, Lauer MS. The prognostic value of estimated creatinine clearance alongside functional capacity in ambulatory patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;40:1106–1113. doi: 10.1016/s0735-1097(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 5.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 6.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 7.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 9.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 10.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 12.Cowburn PJ, Patel H, Jolliffe RE, Wald RW, Parker JD. Cardiac resynchronization therapy: an option for inotrope-supported patients with end-stage heart failure? Eur J Heart Fail. 2005;7:215–217. doi: 10.1016/j.ejheart.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Fung JW, Szeto CC, Chan JY, Zhang Q, Chan HC, Yip GW, et al. Prognostic value of renal function in patients with cardiac resynchronization therapy. Int J Cardiol. 2007;122:10–16. doi: 10.1016/j.ijcard.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Abraham WT. Rationale and design of a randomized clinical trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with advanced heart failure: the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) J Card Fail. 2000;6:369–380. doi: 10.1054/jcaf.2000.20841. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine (abstract) J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 18.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 19.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 21.Burnett JC, Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–F282. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 22.Rodeheffer RJ, Lerman A, Heublein DM, Burnett JC., Jr Increased plasma concentrations of endothelin in congestive heart failure in humans. Mayo Clin Proc. 1992;67:719–724. doi: 10.1016/s0025-6196(12)60795-2. [DOI] [PubMed] [Google Scholar]
- 23.Smilde TD, Hillege HL, Navis G, Boomsma F, de Zeeuw D, van Veldhuisen DJ. Impaired renal function in patients with ischemic and nonischemic chronic heart failure: association with neurohormonal activation and survival. Am Heart J. 2004;148:165–172. doi: 10.1016/j.ahj.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Cowburn PJ, Patel H, Pipes RR, Parker JD. Contrast nephropathy post cardiac resynchronization therapy: an under-recognized complication with important morbidity. Eur J Heart Fail. 2005;7:899–903. doi: 10.1016/j.ejheart.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003;41:765–770. doi: 10.1016/s0735-1097(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 26.Knaapen P, van Campen LM, de Cock CC, Gotte MJ, Visser CA, Lammertsma AA, et al. Effects of cardiac resynchronization therapy on myocardial perfusion reserve. Circulation. 2004;110:646–651. doi: 10.1161/01.cir.0000138108.68719.c1. [DOI] [PubMed] [Google Scholar]
- 27.Lindner O, Vogt J, Kammeier A, Wielepp P, Holzinger J, Baller D, et al. Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur Heart J. 2005;26:70–76. doi: 10.1093/eurheartj/ehi046. [DOI] [PubMed] [Google Scholar]
- 28.Lindner O, Sorensen J, Vogt J, Fricke E, Baller D, Horstkotte D, et al. Cardiac efficiency and oxygen consumption measured with 11C-acetate PET after long-term cardiac resynchronization therapy. J Nucl Med. 2006;47:378–383. [PubMed] [Google Scholar]
- 29.Steendijk P, Tulner SA, Bax JJ, Oemrawsingh PV, Bleeker GB, van Erven L, et al. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation. 2006;113:1295–1304. doi: 10.1161/CIRCULATIONAHA.105.540435. [DOI] [PubMed] [Google Scholar]
- 30.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 31.Saxon LA, De Marco T, Schafer J, Chatterjee K, Kumar UN, Foster E. Effects of long-term biventricular stimulation for resynchronization on echocardiographic measures of remodeling. Circulation. 2002;105:1304–1310. doi: 10.1161/hc1102.105730. [DOI] [PubMed] [Google Scholar]
- 32.Grassi G, Vincenti A, Brambilla R, Trevano FQ, Dell'Oro R, Ciro A, et al. Sustained sympathoinhibitory effects of cardiac resynchronization therapy in severe heart failure. Hypertension. 2004;44:727–731. doi: 10.1161/01.HYP.0000144271.59333.a7. [DOI] [PubMed] [Google Scholar]
- 33.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 34.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108:266–269. doi: 10.1161/01.CIR.0000083368.75831.7A. [DOI] [PubMed] [Google Scholar]
- 35.Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827–1834. doi: 10.1093/eurheartj/ehm192. [DOI] [PubMed] [Google Scholar]
- 36.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]