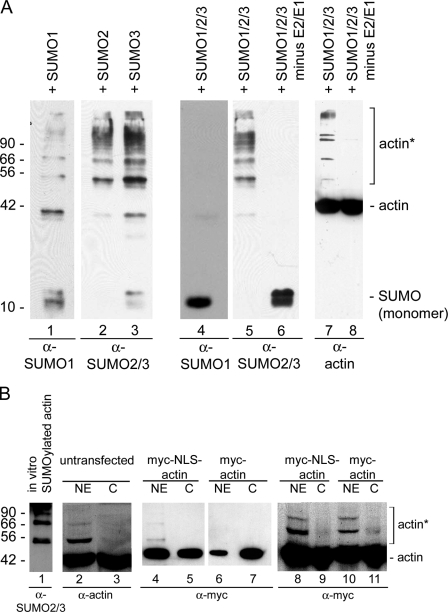
Figure 1.
β-Actin is SUMOylated in vitro. (A) Purified β-actin was incubated with SUMO1, -2, or -3 individually (lanes 1–3) or with all three SUMO proteins (lanes 4–8), and probed with SUMO antibodies (lanes 1–6, bottom) and actin antibodies (lanes 7 and 8). SUMO2 and/or -3 modify actin (lanes 5 and 7), but SUMO1 does not (lane 4), when incubated together. Actin is not modified in the absence of the E1 SUMO-activating and E2-conjugating enzymes (lanes 6 and 8). (B) Nuclear (NE) and cytoplasmic (C) extracts were prepared from HeLa cells, and Western blots were probed with the 56-4 antibody to actin (lanes 2 and 3). Actin was recognized by the actin antibody in the C and NE. A strong band at ∼55 kD and weaker, higher molecular weight bands that co-migrate with actin SUMOylated in vitro were also recognized in the NE by the actin antibody. Immunoblot analyses on HeLa nuclear (NE) and cytoplasmic (C) fractions from cells expressing myc-actin and myc-NLS-actin (lanes 4–11) demonstrated the presence of higher molecular weight bands predominantly in the NE. Equal amounts of protein were applied to lanes 4–7, and lanes 8–11 were loaded with equal amounts of myc-tagged actin. Molecular mass markers are indicated in kD; asterisks, SUMOylated actin in A and B. Purified BSA and β-actin were used as markers in B.