Abstract
Objective
To determine time trends in incidence, prevalence, and clinical characteristics of psoriatic arthritis (PsA) over a 30-year period.
Methods
We identified a population-based incidence cohort of subjects aged 18 or over who fulfilled ClASsification of Psoriatic ARthritis (CASPAR) criteria for PsA between 1/1/1970 and 12/31/1999 in Olmsted County, Minnesota. PsA incidence date was defined as the diagnosis date of those who fulfilled CASPAR criteria. Age- and sex-specific incidence rates were estimated and age- and sex-adjusted to 2000 U.S. white population.
Results
The PsA incidence cohort comprised 147 adult subjects with a mean age of 42.7 years and 61% were males. The overall age- and sex-adjusted annual incidence of PsA per 100,000 was 7.2 (95% CI 6.0, 8.4) with a higher incidence in males (9.1, 95% CI 7.1, 11.0) than females (5.4, 95% CI 4.0, 6.9). The age and sex-adjusted incidence of PsA per 100,000 increased from 3.6 (95% CI 2.0, 5.2) between 1970-79 to 9.8 (95% CI 7.7, 11.9) between 1990-2000 (p for trend < 0.001). The point prevalence per 100,000 was 158 (95% CI 132, 185) in 2000 with a higher prevalence in males (193, 95% CI 150, 237) than females (127, 95% CI 94, 160). At incidence, most PsA subjects had oligoarticular involvement (49%) with enthesopathy (29%).
Conclusions
The incidence of PsA has been rising over thirty years in males and females. Reasons for the increase are unknown, but may be related to a true change in incidence or greater physician awareness of the diagnosis.
Keywords: Epidemiology, Psoriatic Arthritis, Population-based study
INTRODUCTION
Few population-based studies exist documenting the incidence and prevalence of psoriatic arthritis (PsA).(1) Annual incidence estimates of PsA range from 3 to 23 per 100,000 while prevalence estimates vary widely from 0.02% to 0.25% with higher estimates in recent years.(1-13) These variations may be due to secular trends in epidemiology of PsA, increased recognition of the condition over time, or differences in case ascertainment methods in individual studies.(1, 8, 12) Several classification criteria with widely variable sensitivity and specificity have been used, but none have been universally accepted. Hence, the comparability of the published incidence and prevalence estimates of PsA is problematic.
The recently validated classification criteria for PsA by the ClASsification of Psoriatic ARthritis (CASPAR) group offers a unique opportunity to assess time trends in incidence and prevalence rates of PsA using the same criteria consistently over time, irrespective of patients’ assigned diagnoses at the time of their medical care.(14) Furthermore, the sensitivity and specificity of the CASPAR criteria are high in established and early PsA.(14, 15) Therefore, criteria can readily be applied retrospectively to ascertain PsA subjects through review of the original medical records.
In addition, the clinical manifestations of PsA have been heterogenous in reported case series.(8, 12) One reason may be inclusion of subjects with established PsA. The disease course of early PsA differs from late PsA given the tendency of an oligoarticular pattern to evolve into a polyarticular pattern.(8) In this study, we sought to determine the time trends in incidence, prevalence, and the clinical and radiographic features of PsA over a thirty-year period using the recently-validated CASPAR criteria.
METHODS
Study setting
This study was designed as a retrospective, population-based study in Olmsted County, Minnesota using the data resources of the Rochester Epidemiology Project. According to 2000 Olmsted County census, the total population is 124,277, and 90% of individuals are white. The socioeconomic characteristics of the source population is similar to US whites generally, except that a higher percentage of the population is employed in healthcare-related services with a high proportion of college or advanced degrees. The Rochester Epidemiology Project is a diagnostic indexing and medical records linkage system and its potential for population-based studies has been described previously in detail.(16, 17) Population-based, epidemiologic research in this community is enhanced due to relative geographic isolation from urban centers. In addition, nearly all medical care is delivered to local residents by a small number of health care providers, including the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Center, the Olmsted Community Hospital, local nursing homes, and a few private practitioners. All medical, surgical and histological diagnoses and other key information are abstracted and entered into computerized indices to facilitate case identification. This unique population-based data resource ensures virtually complete ascertainment and follow-up of all clinically diagnosed cases of PsA in a geographically-defined community. Furthermore, data from all providers are contained in a single, comprehensive medical records linkage system. Availability of the original medical records, including results of all laboratory and radiographic tests, allows identification and reclassification of potential PsA subjects based on contemporary diagnostic criteria.
Data collection
Using the resources of the Rochester Epidemiology Project, we identified all subjects aged 18 years and over with a diagnosis suggestive of psoriasis and/or psoriatic arthritis from January 1, 1969 through December 31, 1999. The complete medical records (from all healthcare providers) of all subjects were identified and reviewed by a clinical rheumatology fellow (FCW) using a standardized, computerized, pre-tested data abstraction form. Data collection included information on demographics, clinical manifestations, laboratory data and radiographic characteristics. For inclusion into the study as an incident PsA subject, subjects must have demonstrated inflammatory articular disease (joint, spinal, entheseal) documented by either a primary care physician or a rheumatologist and have met the CASPAR criteria(14) at that visit by attaining a score of 3 or greater among the following five categories: 1) current psoriasis (score of 2), a personal history of psoriasis, or a family history of psoriasis; 2) nail dystrophy such as onycholysis, pitting, hyperkeratosis; 3) a negative rheumatoid factor; 4) current dactylitis or a personal history of dactylitis as recorded by a rheumatologist; 5) radiographic evidence of psoriatic bone changes of the hand or foot such as juxtaarticular new bone formation on plain films.(14) The final incidence cohort was comprised of subjects aged ≥ 18 years who first fulfilled CASPAR criteria between January 1, 1970 through December 31, 1999. Subjects who did not fulfill CASPAR criteria or who were diagnosed outside the study time period were excluded. All subjects were followed-up until 1/1/2007 for vital status.
Statistical analysis
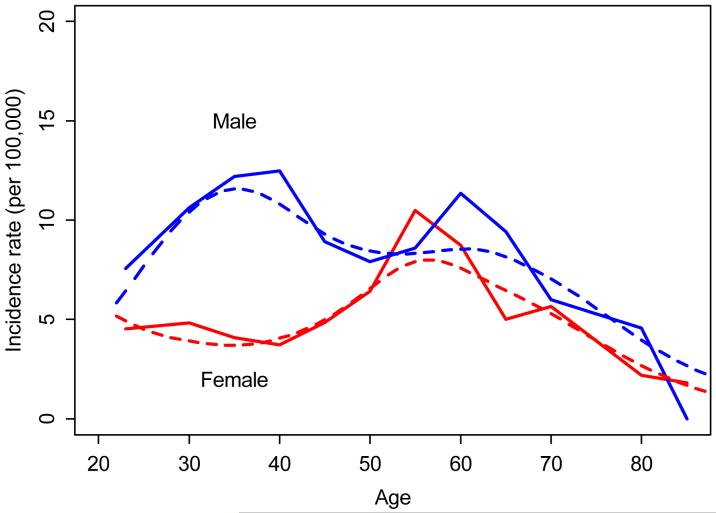
Age-and sex-specific incidence rates were calculated assuming individuals aged 18 and over were at risk for PsA. Age- and sex-specific incidence rates were calculated by using the number of incident cases as the numerator and population estimates based on decennial census counts as the denominator with linear interpolation between census years as described previously.(18) Only subjects who were residents of Olmsted County at disease onset and who fulfilled the CASPAR criteria for PsA were included in the incidence calculations. Overall incidence rates were age- and sex-adjusted to the United States (US) white population. Ninety-five percent confidence intervals (95% CIs) for the incidence rates were constructed using the assumption that the number of incident cases per year follows a Poisson distribution. Incidence trends were examined using Poisson regression models. Smoothing splines were used to model the age effect and obtain the smoothed curves on Figure 1. The prevalence of PsA in 2000 was estimated by using the number of prevalence cases on 1/1/2000 as the numerator and the Olmsted County population in 2000 as the denominator. We accepted a p-value of less than 0.05 as significant for data analysis.
Figure 1. Annual incidence (per 100,000) of PsA by age and sex (1/1/1970-12/31/1999, Olmsted County, Minnesota).
Dotted lines represent smoothed incidence curves obtained using smoothing splines
Kaplan-Meier methods were used to estimate survival. Expected survival in the general population was obtained from the Minnesota white population life tables, according to the age and sex distribution of the PsA cohort. A one-sample log rank test was used to compare observed and expected survival. Age and sex specific incidence and prevalence rates were applied to the total US population in 2000 to estimate the number of affected individuals in the US population.
RESULTS
We identified a total of 357 Olmsted County residents with a potential diagnosis of PsA. Of these, 147 subjects aged ≥18 years fulfilled the CASPAR criteria for inclusion into the study and comprised the final PsA incidence cohort. Reasons for exclusion of the remaining subjects were as follows: did not fulfill CASPAR criteria (128, 35.9%), prevalent PsA subjects with disease onset outside of Olmsted County or disease onset before/after the study time period, or age< 18 years (62, 17.4%), subjects who were not residents of Olmsted County (19, 5.3%), and subject refusal of authorization to use medical records for research (1, 0.3%).
Table 1 shows the demographic characteristics of the 147 incident PsA subjects according to decades of PsA incidence. Overall, there were 90 males (61%). The mean age at incidence was 42 years (±14) for males and 44 years (±17) for females. We did not observe significant trends in gender distribution or the mean age of the incident PsA subjects over time. Although mean age at incidence appeared to have declined over time, this was not significant.
Table 1.
Characteristics of 145 subjects with psoriatic arthritis (PsA) between 1/1/1970 and 12/31/1999 in Olmsted County, Minnesota*
| Time period | ||||
|---|---|---|---|---|
| 1970-1979 | 1980-1989 | 1990-1999 | Total | |
| Total no. (%) of subjects Male Female |
20 12 (60%) 8 (40%) |
46 28 (61%) 18 (39%) |
81 50 (62%) 31 (38%) |
147 90 (61) 57 (39) |
| Age at incidence, mean (± SD) Male Female |
44.1± 18.3 42.8 ± 15.7 46.0 ± 22.7 |
43.6 ± 17.8 44.3 ± 18.4 42.6 ± 17.4 |
41.8 ± 12.8 39.9 ± 10.1 44.8 ± 15.9 |
42.7 ± 15.2 41.7 ± 13.9 44.3 ± 17.1 |
PsA classified according to the CASPAR criteria.
SD, standard deviation
Incidence of PsA
Figure 1 and Table 2 illustrate the age- and sex-specific annual incidence of PsA. The overall age- and sex-adjusted annual incidence of PsA was 7.2 per 100,000 (95% CI 6.0, 8.4). The overall age-adjusted incidence in males (9.1 per 100, 000, 95% CI 7.1, 11.0) was higher than in females (5.4 per 100,000, 95% CI 4.0, 6.9). In males, the annual incidence was highest in the 30-39 age range (12.2 per 100,000) and declined thereafter. In females, the annual incidence was highest in the 50-59 age range (10.5per 100,000) and then declined thereafter. After the sixth decade, incidence in females was similar to the incidence in males (see Figure 1).
Table 2.
Annual incidence (per 100,000) of psoriatic arthritis (PsA) by age and sex between 1/1/1970 and 12/31/1999 in Olmsted County, Minnesota
| Age group | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| No. | Rate | No. | Rate | No. | Rate | |
| 20-29 | 20 | 7.6 | 14 | 4.5 | 34 | 5.9 |
| 30-39 | 29 | 12.2 | 10 | 4.1 | 39 | 8.1 |
| 40-49 | 17 | 9.5 | 9 | 4.9 | 26 | 7.1 |
| 50-59 | 11 | 8.6 | 14 | 10.5 | 25 | 9.6 |
| 60-69 | 8 | 9.4 | 5 | 5.0 | 13 | 7.0 |
| 70-79 | 5 | 10.0 | 4 | 5.2 | 9 | 7.1 |
| 80+ | 0 | 0 | 1 | 1.8 | 1 | 1.3 |
| Total | 90 | 9.1 (7.1, 11.0) † | 57 | 5.4 (4.0, 6.9) † | 147 | 7.2 (6.0, 8.4) ‡ |
PsA classified according to the CASPAR criteria
Age-adjusted to the 2000 United States white population.
Age- and sex-adjusted to the 2000 United States white population.
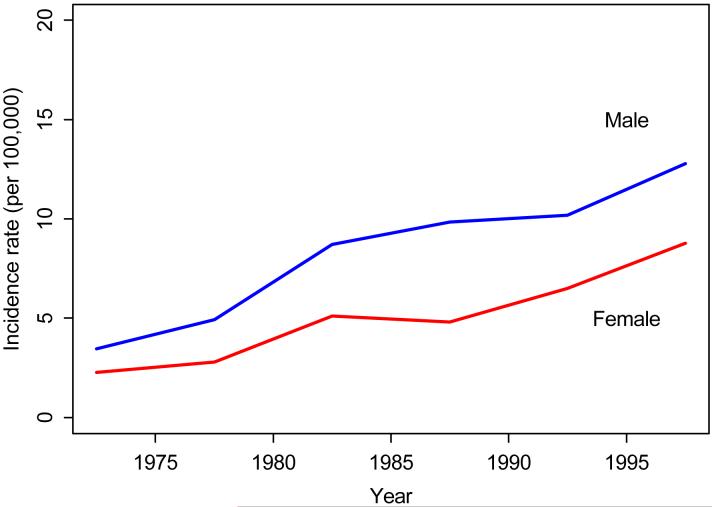
Figure 2 and Table 3 illustrate the overall age-and sex-adjusted incidence over 3 decades. During the 1970-1979 time period, the incidence of PsA per 100,000 was 3.6 (95% CI 2.0, 5.2). The incidence increased to 7.0 per 100,000 (95% CI 4.9, 9.1) between 1980-1989 and 9.8 per 100,000 (95% CI 7.6, 11.9) between 1990-1999. This increasing trend was apparent in both males and females and statistically significant (p<0.001 for trend). We also examined survival in this population in comparison to expected survival. The survival of PsA subjects did not differ from that of the general population (p = 0.66).
Figure 2. Annual incidence (per 100,000) of PsA by calendar year and sex (1/1/1970-12/31/1999, Olmsted County, Minnesota).
Table 3.
Time trends in annual incidence (per 100,000) of psoriatic arthritis (PsA) between 1/1/1970-12/31/1999 in Olmsted County, Minnesota*
| Time period | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| No. | Rate | No. | Rate | No. | Rate | |
| 1970-1979 | 12 | 4.4 (1.9, 7.0) | 8 | 2.6 (0.7, 4.5) | 20 | 3.6 (2.0, 5.2) |
| 1980-1989 | 28 | 9.2 (5.6, 12.9) | 18 | 5.0 (2.6, 7.4) | 46 | 7.0 (4.9, 9.1) |
| 1990-1999 | 50 | 11.8 (8.5, 15.1) | 31 | 7.6 (4.9, 10.3) | 81 | 9.8 (7.6, 11.9) |
PsA classified according to the CASPAR criteria.
Age-adjusted to the 2000 United States white population.
Age- and sex-adjusted to the 2000 United States white population.
Prevalence of PsA
The overall, estimated point prevalence of PsA on 1/1/2000 was 158 per 100,000 (95% CI 132, 185). The sex-specific prevalence per 100,000 was 193 for males (95% CI 150, 237) and 127 for females (95% CI 94, 160).
Clinical characteristics of incident PsA subjects
The clinical characteristics of the PsA subjects at incidence are shown in Table 4. A total of 126 (86%) subjects presented with inflammatory joint disease, followed by enthesopathy in 43 (29%) subjects, and inflammatory back pain in 12 (8%) subjects. The most common enthesopathy sites were in the plantar fascia (28%), finger flexor tendons (28%) and shoulder (21%). Some PsA patients presented with a combination of inflammatory sites (9, 21%). At PsA incidence, 49% of subjects had oligoarticular and 39% had polyarticular involvement. Asymmetrical joint pattern of incident PsA was more common (78%) than symmetric involvement (22%).
Table 4.
Clinical characteristics of 147 incident psoriatic arthritis (PsA) subjects between 1/1/1970-12/31/1999 in Olmsted County, Minnesota*
| Total, N (%) | |
|---|---|
|
Inflammatory articular disease Joint Enthesitis Spine |
126 (86) 43 (29) 12 (8) |
|
Evidence of psoriasis Current Personal history Family history |
138 (94) 8 (5) 30 (21) |
|
Clinical features Nail dystrophy Negative rheumatoid factor† Dactylitis** |
58 (39) 122 (98) 33 (31) |
|
Radiographic findings† Erosions Osteolysis Erosions at DIP Bilateral Sacroiliitis Periostitis Unilateral Sacroiliitis |
42 (32) 17 (13) 14 (11) 9 (7) 5 (4) 4 (3) |
PsA classified according to the CASPAR criteria.
Information not available for 23 subjects.
Information not available for 14 subjects.
Information not available for 39 subjects
The majority of PsA subjects (138, 94%) had psoriasis at PsA incidence. Additionally, 30 (21%) subjects had a family history of psoriasis. However, a family history of psoriasis was not consistently documented in the medical records (60% missing). There were 122 (98%) subjects with a negative rheumatoid factor test among the 124 subjects who received rheumatoid factor testing. Nail dystrophy was present in 40% of the subjects. Out of 108 subjects with documentation, active dactylitis was present in 33 (24%) subjects.
A total of 133 subjects had radiographs, and 73 (55%) subjects had findings consistent with inflammatory joint disease. There were 44 (33%) subjects with evidence of radiological features typical of PsA in hand and wrist radiographs. The most common radiographic changes were erosions in 42 (32%) subjects, including erosions at the distal interphalangeal (DIP) joints in 14 subjects (11%). Seventeen subjects had osteolysis and 5 subjects had periostitis. Additionally, 12 (10.7%) subjects had radiological findings of inflammatory spinal disease. Four subjects had unilateral sacroiliitis and 9 subjects had bilateral sacroiliitis.
DISCUSSION
We are the first to report on the time trends in the incidence and prevalence of PsA using the CASPAR criteria over a 30-year period in a geographically-defined population. Our findings indicate that there is a progressive increase in the incidence of PsA between 1970 and 2000. We have estimated that by the year 2000, the age- and sex-adjusted incidence rate for PsA was 10 per 100,000 with a prevalence of 158 per 100,000. Using these findings, we have extrapolated that between 162,000 to 589,000 adults aged ≥ 18 years were affected with PsA in the US in 2000, and 8,000 to 27,000 new cases occur each year.
There are only a limited number of incidence studies of PsA. (1, 3, 6, 7, 12) This is the first study that examines PsA incidence trends using the same classification criteria consistently over an extended period. Although our PsA incidence estimates are broadly consistent with previous estimates, the most significant finding is the increasing incidence over time. This is in contrast to rheumatoid arthritis, a Th1-mediated disease, which has shown a decline in incidence over the same time period.(19) Previous studies have suggested a birth-cohort effect to explain the decline in rheumatoid arthritis with a higher incidence at the turn of the century, suggesting infectious or environmental triggers(20). Reasons for the change in PsA incidence are also unclear, but may be due to greater physician awareness, the changing nature of the disease, or unknown environmental or genetic risk factors.(21-24) Of note, the incidence of psoriasis also has increased in this population during the same time period(25) and thus the increase in incidence of PsA may parallel the increasing psoriasis trend.
Intriguingly, our findings also demonstrate a difference in the age-specific incidence of PsA between men and women. Contrary to prevailing belief that PsA occurs equally in both sexes, our findings indicate that the incidence of PsA in women is less than the incidence in men until the sixth decade of life.(8, 12) A similar pattern was observed in the incidence of psoriasis in Olmsted County population and in a recent database study from the United Kingdom.(25, 26) These findings suggest a possible hormonal influence in the onset of PsA. Pregnancy has been associated with a reduced likelihood of PsA.(23) Furthermore, PsA improves during pregnancy, but disease flares are common during the post-partum period when estrogen levels are in flux. (27-30) Also, patients treated with estrogen-modifying drugs were less likely to develop PsA.(21) Although evidence is limited in PsA, research in rheumatoid arthritis and systemic lupus erythematosus has demonstrated that changes in cortisol, estrogen, progesterone and glucocorticoids levels, and androgen/estrogen balance results in a down-regulation of the Th1 response and a shift toward a Th2 cytokine profile.(31) In summary, our gender-specific incidence patterns provide indirect evidence of the potential role of sex hormones in the etiology of PsA. Further research is needed to elucidate the complex role of sex hormones in the pathogenesis of PsA in men and women.
Our study also provides further evidence on the clinical and radiographic features in early PsA. First, most subjects in our incidence cohort satisfied CASPAR criteria based on the presence of psoriasis and a negative rheumatoid factor. This is consistent with patterns seen at other centers.(15) Second, our study demonstrates an oligoarticular pattern with predominance of enthesopathy, similar to other early PsA case series. (32, 33) In contrast, studies of prevalent PsA subjects have documented the predominance of polyarticular involvement.(34-36) As suggested by Gladman et al, PsA begins as an oligoarticular disease that may evolve into a polyarticular phenotype.(8) Third, a common radiographic feature in our incidence cohort was erosions, especially at the distal interphalangeal joints. Uncommon findings were osteolysis, fluffy periostitis, and bony proliferation or ankylosis. Reasons for the relative lack of radiographic joint severity may be because our subjects are ascertained during the early stages of PsA. Prevalent PsA subjects may have prolonged exposure to the pro-inflammatory cytokines that are responsible for promoting the cascade that leads to bone destruction and new bone formation.(24, 37) Alternatively, erosive disease or severe radiographic changes of bony deformation are more frequent in PsA subjects who have symmetrical, polyarticular disease, a group not common in our study.(8, 38, 39)
In contrast to previous studies(40, 41), we did not observe an increase in overall mortality in our incidence cohort of PsA patients. This was also reported by an earlier study in this population(3). As recently addressed by Ali and colleagues(42), a few reasons for the discrepant findings may be an early, less severe stage of the disease in our incidence cohort with the potential for exposure to sustained treatment that dampens the inflammatory state in later years.
Our study may have several potential limitations. First, because of the variable disease course, there is the possibility that we missed PsA cases that presented with minimal or no psoriatic skin lesions, as inflammatory arthritis may precede the diagnosis of PsA in approximately 15-19% of subjects(35, 43). The sensitivity of the CASPAR criteria is only 91% and therefore, some true PSA cases may not have fulfilled CASPAR criteria. However, over the course of 30 years, we expected that most PsA cases and psoriasis lesions would have been ascertained due to the comprehensive medical records linkage system provided by the Rochester Epidemiology Project.(16) Second, there is the concern of reviewer/detection bias due to the retrospective nature of the study. However, the majority of data abstraction was performed with a standardized, pre-tested data abstraction form that included explicit variable definitions provided by the CASPAR criteria.(14) Questions regarding individual PsA diagnosis were discussed with the other co-investigators and resolved by consensus. Third, the availability of information in the medical records may be subject to clinician variability; hence, information on clinical variables, such as nail pitting or the number of affected joints, may have been incompletely documented. Similarly, radiographic results in the earlier years of the study were available only as paper copies with reports of “erosions typical of psoriatic arthritis” without further elaboration. Hard copies of radiographic films were not reviewed for detailed confirmation of the presence or absence of individual radiographic features. Nevertheless, radiographic evidence was not necessary if a patient already accumulated 3 points to achieve the CASPAR criteria. Finally, the population of Olmsted County, Minnesota is primarily white (90%) and thus limits the generalization of our incidence estimates to racially-diverse populations.
Nevertheless, strengths of our study include the unique medical records linkage system provided by the Rochester Epidemiology Project that allows for near-complete ascertainment of all clinically-recognized cases of PsA in a well-defined population. In addition, this is the first study that examines not only the time trends in the epidemiology of PsA, but also relies on the newly-validated CASPAR criteria that previously has been shown to have a sensitivity of 91.4% and sensitivity of 98.7%.(14) The CASPAR criteria thus can be used in retrospective studies, provided that documentation of clinical and radiographic characteristics is available.
In conclusion, our findings indicate that the incidence of PsA has been rising over 30 years in males and females. Incidence patterns in males and females follow different patterns, suggesting the potential role of sex hormones in the development of PsA. Further research is needed to elucidate the reasons for the increase in PsA incidence and the gender-specific patterns, including the potential role of environmental and hormonal risk factors.
ACKNOWLEDGEMENT
We wish to acknowledge Mitch Bais for his assistance in obtaining medical records, David Tines for preparing the data abstraction screens, and Justin Carlin for data management.
Footnotes
Conflict of interest: Funded by an unrestricted research grant from Amgen Inc.
References
- 1.Alamanos Y, Voulgari PV, Drosos AA. Incidence and Prevalence of Psoriatic Arthritis: A Systematic Review. J Rheumatol. 2008 [PubMed] [Google Scholar]
- 2.Hellgren L. Association between rheumatoid arthritis and psoriasis in total populations. Acta Rheumatol Scand. 1969;15(4):316–26. doi: 10.3109/rhe1.1969.15.issue-1-4.40. [DOI] [PubMed] [Google Scholar]
- 3.Shbeeb M, Uramoto KM, Gibson LE, O’Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982-1991. J Rheumatol. 2000;27(5):1247–50. [PubMed] [Google Scholar]
- 4.Soderlin MK, Borjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis. 2002;61(10):911–5. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savolainen E, Kaipiainen-Seppanen O, Kroger L, Luosujarvi R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol. 2003;30(11):2460–8. [PubMed] [Google Scholar]
- 6.Kaipiainen-Seppanen O. Incidence of psoriatic arthritis in Finland. Br J Rheumatol. 1996;35(12):1289–91. doi: 10.1093/rheumatology/35.12.1289. [DOI] [PubMed] [Google Scholar]
- 7.Kaipiainen-Seppanen O, Aho K. Incidence of chronic inflammatory joint diseases in Finland in 1995. J Rheumatol. 2000;27(1):94–100. [PubMed] [Google Scholar]
- 8.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4) doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol. 2007;34(10):2082–8. [PubMed] [Google Scholar]
- 10.De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol. 2007;36(1):14–21. doi: 10.1080/03009740600904243. [DOI] [PubMed] [Google Scholar]
- 11.Alamanos Y, Papadopoulos NG, Voulgari PV, Siozos C, Psychos DN, Tympanidou M, et al. Epidemiology of psoriatic arthritis in northwest Greece, 1982-2001. J Rheumatol. 2003;30(12):2641–4. [PubMed] [Google Scholar]
- 12.Setty AR, Choi HK. Psoriatic arthritis epidemiology. Curr Rheumatol Rep. 2007;9(6):449–54. doi: 10.1007/s11926-007-0073-3. [DOI] [PubMed] [Google Scholar]
- 13.Madland TM, Apalset EM, Johannessen AE, Rossebo B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol. 2005;32(10):1918–22. [PubMed] [Google Scholar]
- 14.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 15.Chandran V, Schentag CT, Gladman DD. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum. 2007;57(8):1560–3. doi: 10.1002/art.23104. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004:819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder D, Offord K. A SAS macro which utilizes local and reference population counts appropriate for incidence, prevalence, and mortality rate calculations in Rochester and Olmsted County. Mayo Clinic; Minnesota. Rochester, MN: 1982. [Google Scholar]
- 19.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 20.Enzer I, Dunn G, Jacobsson L, Bennett PH, Knowler WC, Silman A. An epidemiologic study of trends in prevalence of rheumatoid factor seropositivity in Pima Indians: evidence of a decline due to both secular and birth-cohort influences. Arthritis Rheum. 2002;46(7):1729–34. doi: 10.1002/art.10360. [DOI] [PubMed] [Google Scholar]
- 21.Pattison EJ, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case control study. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.073932. [DOI] [PubMed] [Google Scholar]
- 22.Queiro R, Torre JC, Gonzalez S, Lopez-Larrea C, Tinture T, Lopez-Lagunas I. HLA antigens may influence the age of onset of psoriasis and psoriatic arthritis. J Rheumatol. 2003;30(3):505–7. [PubMed] [Google Scholar]
- 23.Thumboo J, Uramoto K, Shbeeb MI, O’Fallon WM, Crowson CS, Gibson LE, et al. Risk factors for the development of psoriatic arthritis: a population based nested case control study. J Rheumatol. 2002;29(4):757–62. [PubMed] [Google Scholar]
- 24.Anandarajah AP, Ritchlin CT. Pathogenesis of psoriatic arthritis. Curr Opin Rheumatol. 2004;16(4):338–43. doi: 10.1097/01.bor.0000129718.13939.81<. [DOI] [PubMed] [Google Scholar]
- 25.Icen M, Maradit Kremers H, Crowson CS, McEvoy MT. Trends in incidence of psoriasis over three decades: a population based study[abstract] American Academy of Dermatology 2008 Annual Meeting. J Am Acad Dermatol. 2007;58(2):AB78. [Google Scholar]
- 26.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–65. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 27.McNeill ME. Multiple pregnancy-induced remissions of psoriatic arthritis: case report. Am J Obstet Gynecol. 1988;159(4):896–7. doi: 10.1016/s0002-9378(88)80165-0. [DOI] [PubMed] [Google Scholar]
- 28.Ostensen M. Pregnancy in psoriatic arthritis. Scand J Rheumatol. 1988;17(1):67–70. doi: 10.3109/03009748809098763. [DOI] [PubMed] [Google Scholar]
- 29.McHugh NJ, Laurent MR. The effect of pregnancy on the onset of psoriatic arthritis. Br J Rheumatol. 1989;28(1):50–2. doi: 10.1093/rheumatology/28.1.50. [DOI] [PubMed] [Google Scholar]
- 30.Ostensen M. The effect of pregnancy on ankylosing spondylitis, psoriatic arthritis, and juvenile rheumatoid arthritis. Am J Reprod Immunol. 1992;28(34):235–7. doi: 10.1111/j.1600-0897.1992.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 31.Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29(2):185–91. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 32.Scarpa R, Cuocolo A, Peluso R, Atteno M, Gisonni P, Iervolino S, et al. Early psoriatic arthritis: the clinical spectrum. J Rheumatol. 2008;35(1):137–41. [PubMed] [Google Scholar]
- 33.Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42(12):1460–8. doi: 10.1093/rheumatology/keg384. [DOI] [PubMed] [Google Scholar]
- 34.Wright V, Roberts MC, Hill AG. Dermatological manifestations in psoriatic arthritis: a follow-up study. Acta Derm Venereol. 1979;59(3):235–40. [PubMed] [Google Scholar]
- 35.Scarpa R, Oriente P, Pucino A, Torella M, Vignone L, Riccio A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol. 1984;23(4):246–50. doi: 10.1093/rheumatology/23.4.246. [DOI] [PubMed] [Google Scholar]
- 36.Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;64(Suppl 2):ii3–8. doi: 10.1136/ard.2004.032318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hueber AJ, McInnes IB. Immune regulation in psoriasis and psoriatic arthritis-Recent developments. Immunol Lett. 2007;114(2):59–65. doi: 10.1016/j.imlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Kammer GM, Soter NA, Gibson DJ, Schur PH. Psoriatic arthritis: a clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum. 1979;9(2):75–97. doi: 10.1016/s0049-0172(79)80001-3. [DOI] [PubMed] [Google Scholar]
- 39.Queiro-Silva R, Torre-Alonso JC, Tinture-Eguren T, Lopez-Lagunas I. A polyarticular onset predicts erosive and deforming disease in psoriatic arthritis. Ann Rheum Dis. 2003;62(1):68–70. doi: 10.1136/ard.62.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum. 1998;41(6):1103–10. doi: 10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 41.Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40(10):1868–72. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- 42.Ali Y, Tom BD, Schentag CT, Farewell VT, Gladman DD. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007;56(8):2708–14. doi: 10.1002/art.22800. [DOI] [PubMed] [Google Scholar]
- 43.Leung Y, Lim K. Apsoriatic psoriatic arthritis. APLAR J Rheumatol. 2007;10:264–269. [Google Scholar]