Abstract
Purpose
Recently, a 36-kDa variant of estrogen receptor α (ER-α66), ER-α36, has been identified and cloned. ER-α36 predominantly localizes on the plasma membrane and in the cytoplasm and mediates a membrane-initiated “nongenomic” signaling pathway. Here, we investigate the association between ER-α36 expression and tamoxifen resistance in patients with breast cancer.
Patients and Methods
ER-α36 protein expression in tumors from 896 women (two independent cohorts, 1 and 2) with operable primary breast cancer was assessed using an immunohistochemistry assay.
Results
In the first cohort of 710 consecutive patients, overexpression of ER-α36 was associated with poorer disease-free survival (DFS) and disease-specific survival (DSS) in patients with ER-α66–positive tumors who received tamoxifen treatment (chemotherapy plus tamoxifen or tamoxifen alone, n = 307). In contrast, ER-α36 was not associated with survival in patients with ER-α66–positive tumors who did not receive tamoxifen (chemotherapy alone, n = 129) and in patients with ER-α66–negative tumors whether they received tamoxifen (n = 73) or not (n = 149). In the second cohort of 186 patients who only received tamoxifen as adjuvant therapy, overexpression of ER-α36 was significantly associated with poorer DFS and DSS in 156 ER-α66–positive patients from this cohort, and ER-α36 remained an independent unfavorable factor for both DFS and DSS in these 156 patients by a multivariate analysis (DFS: hazard ratio [HR] = 5.47; 95% CI, 1.81 to 16.51; P =. 003; DSS: HR = 13.97; 95% CI, 1.58 to 123.53; P = .018).
Conclusion
Women with ER-α66–positive tumors that also express high levels of ER-α36 are less likely to benefit from tamoxifen treatment.
INTRODUCTION
Estrogen receptor α (also known as ER-α66) is one of the most important determinants of susceptibility to endocrine therapy in breast cancer. In general, patients with ER-α66–positive breast cancer respond favorably to tamoxifen, and tamoxifen has proved to be effective in the treatment of all stages of ER-α66–positive breast cancers.1,2 However, approximately 40% of ER-α66–positive tumors fail to respond to tamoxifen therapy at diagnosis.3 The exact mechanisms underlying this de novo tamoxifen resistance have not been established. A number of hypotheses have been proposed to explain tamoxifen resistance, including altered pharmacology of tamoxifen, modification of the ER-α66 structure and function, cross-talk between the ER-α66 pathway and growth factor signaling pathways, and altered expression of coactivators and/or corepressors.3–7
Recently, we have identified and cloned a novel variant of ER-α that has a molecular weight of 36-kDa, and thus we have termed it ER-α36.8 The transcript of ER-α36 is initiated from a previously unidentified promoter in the first intron of the ER-α66 gene. ER-α36 differs from ER-α66 by lacking both transcriptional activation domains (AF-1 and AF-2) but retaining the DNA-binding domain and partial dimerization and ligand-binding domains.8 It possesses a unique 27–amino acid domain that replaces the last 138 amino acids encoded by the exon 7 and 8 of the ER-α66 gene. ER-α36 is predominantly expressed on the plasma membrane and in the cytoplasm, where it mediates membrane-initiated effects of estrogen signaling, such as activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway, and stimulates cell growth.9 In ER-α36–overexpressing cells, tamoxifen treatment fails to block the ER-α36–mediated activation of the MAPK/ERK pathway; instead, it stimulates cell growth.9 These findings raise an intriguing possibility that ER-α36 may be involved in de novo tamoxifen resistance in breast cancer. To test this hypothesis, we examined ER-α36 expression in tumor specimens from 896 patients with primary breast cancer (including two independent cohorts, 1 and 2). We aimed to investigate whether ER-α36 expression is associated with the clinical outcome of patients with breast cancer treated with tamoxifen.
PATIENTS AND METHODS
Study Population
In the first cohort, a total of 907 consecutive patients with operable primary breast cancer were treated at Peking University School of Oncology from December 1994 to December 1999. Paraffin blocks of tumor tissue were available for 769 patients. Among these, we failed to assess ER-α36 staining in 59 tumor specimens as a result of tissue loss during slide preparation. Therefore, specimens from 710 patients with operable primary breast cancer in the first cohort were analyzed in this study. To further verify the results from the first cohort, an independent second cohort of patients was included in this study. Approximately 3,260 consecutive patients with operable primary breast cancer were treated at Peking University School of Oncology (including the Breast Center and Surgical Department Units I to IV) from January 2000 to December 2006. Among them, 186 patients with available paraffin blocks received only tamoxifen as their adjuvant therapy after surgery. Tumor size was defined as the maximum tumor diameter measured on the tumor specimens at the time of operation. Patients received radical mastectomy, modified radical mastectomy, or breast-conserving surgery; the axillary lymph nodes were routinely dissected at least at levels I and II, and lymph node metastasis was determined based on histologic examination. The majority of patients in the first cohort received adjuvant chemotherapy alone (cyclophosphamide, methotrexate, and fluorouracil or anthracycline-based regimen) or sequential chemotherapy and endocrine therapy; patients from the first cohort who had ER-α66– and/or progesterone receptor (PgR) –positive tumors usually received adjuvant tamoxifen treatment (20 mg/d) for 5 years after chemotherapy or surgery. Patients from the second cohort received adjuvant tamoxifen treatment (20 mg/d) only after surgery. The follow-up data were available for all patients, with a median follow-up of 7.9 years (range, 0.4 to 11.1 years) for the first cohort; the median follow-up is 4.8 years (range, 0.3 to 8.1) for the second cohort. This study was approved by the Research and Ethical Committee of Peking University School of Oncology.
Hormone Receptors
ER-α66 and PgR expression in 609 tumors from cohort 1 were measured by using a dextran-coated charcoal assay as previously described.10 [3H]-estradiol (Amersham, Buckinghamshire, United Kingdom) and [3H]-R5020 (Dupont New England Nuclear, Boston, MA) were used as the labeled ligands for ER-α66 and PgR analysis, respectively. Specimens containing at least 10 fmol/mg of protein were considered ER-α66 or PgR positive. ER-α66 and PgR expression in the remaining 101 cases from cohort 1 and in the 186 tumors from cohort 2 were assessed by immunohistochemical assay using an ER-α66–specific antibody raised against the N-terminal of ER-α66 epitope (clone: 1D5, Zymed, South Francisco, CA; dilution 1:100) and a PgR specific antibody (clone: 1A6, Zymed; dilution 1:100), respectively. ER-α66 or PgR immunostaining was considered positive when ≥ 10% of tumor cells showed positive nuclear staining.
Immunohistochemistry Assay
Immunohistochemical staining was performed on 4-μm thick tumor sections via a two-step assay. Briefly, tissue slides were deparaffinized with xylene and rehydrated through a graded alcohol series. The endogenous peroxidase activity was blocked by incubation in a 3% hydrogen peroxide/methanol buffer for 10 minutes. Antigen retrieval was carried out by immersing the slides in EDTA buffer (pH 8.0) and boiling in a waterbath at 95°C for 25 minutes. The slides were rinsed in phosphate-buffered saline and incubated with normal goat serum to block nonspecific staining. The slides were then incubated with the primary antibody (a polyclonal anti–ER-α36 antibody raised against the last 20 amino acids as a custom service by Alpha Diagnostic International, San Antonio, TX; dilution 1:50) as described previously9 overnight at 4°C in a humidified chamber. The sections were incubated with the second antibody (horseradish peroxidase-conjugated goat antirabbit immunoglobin; 1:100, Dako, Copenhagen, Denmark) for 45 minutes. Diaminobenzidine was used as a chromogen, and sections were counterstained with hematoxylin. The staining intensity in the cytoplasm and the plasma membrane was evaluated. Scoring for ER-α36 staining was graded as follows: no staining or staining observed in less than 10% of tumor cells was given a score 0; faint/barely perceptible staining detected in ≥ 10% of tumor cells was scored as 1+; a moderate or strong complete staining observed in ≥ 10% of tumor cells was scored as 2+ or 3+, respectively. Scores of 0 and 1+ were considered negative, whereas 2+ and 3+ were considered positive. Human epidermal growth factor receptor 2 (HER-2) expression was determined using an immunohistochemistry assay as described previously using an HER-2 specific antibody (clone CB-11, Zymed; dilution 1:100).11 Only the membrane staining was scored, and a score of 3+ was considered as HER-2 positive. The immunostained slides were evaluated by two pathologists (B.D. and Z.L.) who independently examined the whole slide in a blinded manner. In most cases, the evaluations of the two pathologists were identical; any discrepancies were resolved by re-examination and consensus.
Statistical Analysis
The correlation between ER-α36 expression, clinicopathologic characteristics, and adjuvant tamoxifen treatment was determined using Pearson's χ2 test. Disease-free survival (DFS) was defined as the time from date of diagnosis to first recurrence (local or distant) or death from breast cancer without a recorded relapse. Disease-specific survival (DSS) was defined as the time from date of diagnosis to death where breast cancer was the primary or underlying cause of death. Patients who were alive at the last follow-up were censored at the last follow-up date, and patients who died from causes other than breast cancer were censored at the time of death. Survival curves were derived from Kaplan-Meier estimates, and the curves were compared using log-rank tests. A Cox regression model was applied to determine whether a factor was an independent predictor of survival in multivariate analysis. All statistical tests were two-sided, and P values less than .05 were considered as statistically significant. The statistical analyses were performed using SPSS 13.0 software (SPSS Inc, Chicago, IL).
RESULTS
Clinicopathologic Characteristics
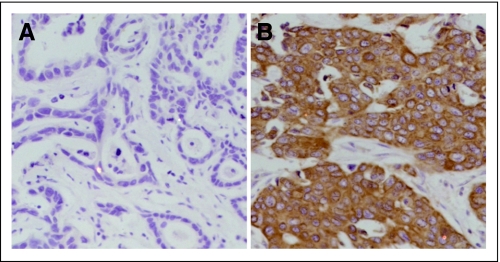
The clinicopathologic characteristics of the two cohorts are presented in Table 1. Unlike ER-α66 that is mainly localized in the cell nucleus, ER-α36 staining was predominantly observed on the plasma membrane and in the cytoplasm (Fig 1). In the first cohort of 710 patients, 39% of patients exhibited high levels of ER-α36 expression in their tumors, whereas the remaining 61% of tumors exhibited a low level of expression (description of cutoff for ER-α36 expression in Patients and Methods). In the second cohort of 186 patients, 46% of patients exhibited high levels of ER-α36, and the remaining 54% showed low levels of expression (Table 1). Thirty-nine percent (182 of 465) of ER-α66–positive and 41% (96 of 237) of ER-α66–negative tumors exhibited a positive ER-α36 staining in the first cohort, respectively (Appendix Table A1, online only). ER-α36 expression was not associated with ER-α66 expression, tumor size, or lymph node status, but was significantly associated with menopausal status and expression of PgR and HER-2 in the first cohort. Patients with ER-α36–positive tumors tended to be older and were more likely to have HER-2–positive and PgR-negative tumors than those with ER-α36–negative tumors in this group (Appendix Table A1). In contrast, no associations were observed between ER-α36 and clinicopathologic characteristics in the second cohort of 186 patients, possibly due to a selection bias (Appendix Table A2, online only).
Table 1.
Characteristics of Cohort 1 and 2
| Characteristic | Cohort 1 |
Cohort 2 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total | 710 | 186 | ||
| ER-α36 status | ||||
| Positive | 280 | 39 | 86 | 46 |
| Negative | 430 | 61 | 100 | 54 |
| Menopausal status | ||||
| Premenopausal | 350 | 49 | 53 | 28 |
| Postmenopausal | 360 | 51 | 133 | 72 |
| Tumor size, cm | ||||
| ≤ 2 | 441 | 62 | 120 | 65 |
| > 2 | 269 | 38 | 66 | 35 |
| Lymph node status | ||||
| 0 | 431 | 61 | 147 | 79 |
| 1-3 | 146 | 20 | 20 | 11 |
| ≥ 4 | 133 | 19 | 19 | 10 |
| ER-α66 status | ||||
| Positive | 465 | 66 | 156 | 85 |
| Negative | 237 | 34 | 27 | 15 |
| Unknown | 8 | 3 | ||
| PgR status | ||||
| Positive | 355 | 50 | 124 | 68 |
| Negative | 349 | 50 | 58 | 32 |
| Unknown | 6 | 4 | ||
| HER-2 status | ||||
| Positive | 142 | 20 | 36 | 20 |
| Negative | 564 | 80 | 149 | 80 |
| Unknown | 4 | 1 | ||
| Histologic grade | ||||
| I | 153 | 23 | 64 | 38 |
| II | 469 | 70 | 99 | 58 |
| III | 49 | 7 | 6 | 4 |
| Unknown | 39 | 17 | ||
| Adjuvant therapy | ||||
| Chemotherapy | 280 | 41 | 0 | |
| Chemotherapy plus tamoxifen | 291 | 42 | 0 | |
| Tamoxifen alone | 91 | 13 | 186 | 100 |
| No treatment | 24 | 4 | 0 | |
| Unknown | 24 | 0 | ||
Abbreviations: ER-α36, estrogen receptor-α 36; ER-α66, estrogen receptor-α 66; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
Fig 1.
Immunohistochemical staining for estrogen receptor (ER)-α36 expression in primary breast cancers. ER-α36 showed (A) negative staining and (B) positive staining (magnification ×200).
ER-α36 Expression and Survival in Cohort 1
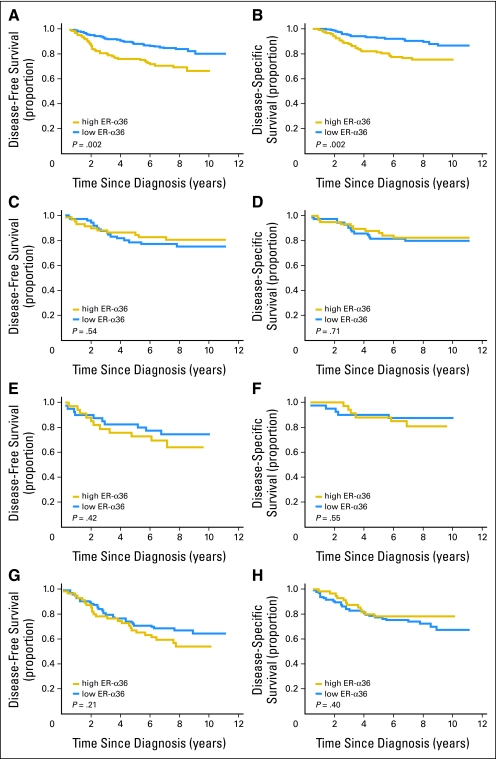
In the first cohort of 710 patients, ER-α36 expression was significantly associated with survival in ER-α66–positive patients; patients with both ER-α66– and ER-α36–positive tumors had poorer DFS and DSS than did those with ER-α66–positive/ER-α36–negative tumors (data not shown). We stratified these 465 ER-α66–positive patients according to adjuvant treatments. Among these, 307 patients received tamoxifen treatment (chemotherapy plus tamoxifen or tamoxifen alone), whereas 129 patients did not receive tamoxifen (chemotherapy alone), the remaining 29 patients either did not receive adjuvant therapy or the adjuvant therapy information was not available. In the 307 patients with ER-α66–positive tumors who received tamoxifen treatment, patients with ER-α36–positive tumors had poorer DFS and DSS than did those with ER-α36–negative tumors (5-year DFS: 76% v 88%, P = .002; and 5-year DSS: 81% v 92%, P = .002, respectively; Figs 2A and 2B). Multivariate analysis revealed that overexpression of ER-α36 was an independent unfavorable factor for both DFS and DSS (DFS: hazard ratio [HR] = 1.92; 95% CI, 1.17 to 3.14; P =. 009; DSS: HR = 2.48; 95% CI, 1.40 to 4.40; P = .002) in these 307 ER-α66–positive patients after adjustment for menopausal status, histologic grade, tumor size, lymph node status, PgR status, and HER-2 status (Table 2). In addition, among these 307 patients, patients with both ER-α36 and HER-2–positive tumors had more unfavorable survival than did patients with ER-α36–positive/HER-2–negative tumors (data not shown). However, this was not the case for patients with ER-α36–positive/PgR-negative tumors as compared with patients with both ER-α36– and PgR-positive tumors. Among the 129 patients with ER-α66–positive tumors who received chemotherapy alone, although ER-α36 expression was not significantly associated with DFS and DSS (Fig 2C and D), overexpression of ER-α36 seemed to be a favorable factor of DFS and DSS in this subgroup in a multivariate analysis (Table 2). Furthermore, when the patients with ER-α66–positive tumors who received tamoxifen as their only adjuvant therapy (n = 79) were analyzed, overexpression of ER-α36 expression was significantly associated with poorer DFS and DSS in this relatively small group (data not shown).
Fig 2.
Kaplan-Meier estimate of disease-free survival (DFS) and disease-specific survival (DSS) in cohort 1, according to estrogen receptor (ER)-α36 expression. (A) DFS and (B) DSS in 307 ER-α66–positive patients who received tamoxifen treatment (chemotherapy plus tamoxifen or tamoxifen alone); (C) DFS or (D) DSS in 129 ER-α66–positive patients who did not receive tamoxifen therapy (chemotherapy alone); (E) DFS or (F) DSS in 73 ER-α66–negative patients who received tamoxifen therapy (chemotherapy plus tamoxifen or tamoxifen alone); (G) DFS or (H) DSS in 149 ER-α66–negative patients who did not receive tamoxifen therapy (chemotherapy alone).
Table 2.
Results of Multivariate Analyses of ER-α36 Positive Versus ER-α36 Negative for DFS and DSS in Four Subgroups in Cohort 1
| Group | No.* | DFS |
DSS |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| ER-α66 positive/TAM+ | 307 | 1.92 | 1.17 to 3.14 | .009 | 2.48 | 1.40 to 4.40 | .002 |
| ER-α66 positive/TAM− | 129 | 0.84 | 0.38 to 1.85 | .67 | 0.79 | 0.34 to 1.83 | .58 |
| ER-α66 negative/TAM+ | 73 | 1.55 | 0.62 to 3.85 | .35 | 1.29 | 0.37 to 4.51 | .69 |
| ER-α66 negative/TAM− | 149 | 0.95 | 0.53 to 1.68 | .86 | 0.52 | 0.25 to 1.05 | .07 |
Abbreviations: ER-α36, estrogen receptor-α 36; DFS, disease-free survival; DSS, disease-specific survival; HR, hazard ratio; ER-α66, estrogen receptor-α 66; TAM+, chemotherapy plus tamoxifen or tamoxifen alone; TAM−, chemotherapy alone without tamoxifen treatment.
Twenty-nine ER-α66–positive patients and 15 ER-α66–negative patients in cohort 1 either did not receive adjuvant therapy or the adjuvant therapy information was not available, and the ER-α66 status is unknown in eight patients in cohort 1.
Among the 237 patients with ER-α66–negative tumors, 15 patients either did not receive adjuvant therapy or the therapy information was not available. The remaining 222 ER-α66–negative tumors were included for survival analysis. ER-α36 expression was not significantly associated with DFS or DSS regardless of whether the patients received tamoxifen treatment (chemotherapy plus tamoxifen or tamoxifen alone, n = 73; Figs 2E and 2F) or not (chemotherapy alone, n = 149; Figs 2G and 2H). However, in 149 ER-α66–negative patients who received chemotherapy alone, overexpression of ER-α36 tended to be a favorable factor of DSS in a multivariate analysis (Table 2).
ER-α36 Expression and Survival in Cohort 2 Who Received Only Tamoxifen Treatment
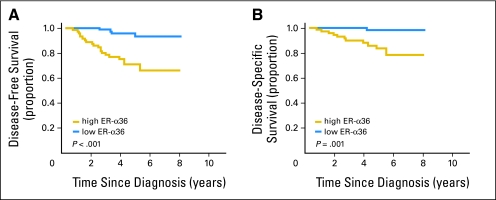
The results from cohort 1 suggested that ER-α36 might be associated with tamoxifen resistance in patients with ER-α66–positive breast cancer who received tamoxifen treatment. To verify this finding, an independent second cohort of 186 patients who received tamoxifen as their only adjuvant therapy was included for analysis. Univariate analysis showed that patients with ER-α36–positive tumors had poorer DFS and DSS than did patients with ER-α36–negative tumors in these 186 patients (data not shown). When the analysis was restricted to patients with ER-α66–positive tumors receiving only tamoxifen in this cohort (n = 156), overexpression of ER-α36 was significantly associated with poorer DFS and DSS (5-year DFS: 71% v 93%, P < .001; and 5-year DSS: 83% v 98%, P = .001, respectively; Figs 3A and 3B). Furthermore, a multivariate analysis revealed that ER-α36 was an independent unfavorable factor for both DFS and DSS (DFS: HR = 5.47; 95% CI, 1.81 to 16.51; P =. 003; DSS: HR = 13.97; 95% CI, 1.58 to 123.53; P = .018) in these 156 patients after adjustment for menopausal status, histologic grade, tumor size, lymph node status, PgR status, and HER-2 status (Table 3). Negative PgR and more than four positive lymph nodes also remained independent unfavorable factors for both DFS and DSS, with more than four positive lymph nodes having the most influence (Table 3).
Fig 3.
Kaplan-Meier estimate of disease-free survival (DFS) and disease-specific survival (DSS) in the 156 estrogen receptor (ER)-α66–positive patients who only received tamoxifen in cohort 2. High levels of ER-α36 expression were significantly associated with poorer (A) DFS and (B) DSS in this group.
Table 3.
Multivariate Analyses of Disease-Free Survival and Disease-Specific Survival in 156 ER-α66–Positive Patients Who Only Received Tamoxifen Treatment in Cohort 2
| Factor | DFS |
DSS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| ER-α36 status, positive v negative | 5.47 | 1.81 to 16.51 | .003 | 13.97 | 1.58 to 123.53 | .018 |
| Menopausal status, post v pre | 3.88 | 0.89 to 16.96 | .07 | 6.21 | 0.67 to 57.35 | .11 |
| Tumor size, > 2 v ≤ 2 cm | 1.34 | 0.42 to 4.32 | .62 | 1.19 | 0.22 to 6.48 | .84 |
| PgR status, negative v positive | 3.31 | 1.32 to 8.31 | .011 | 4.27 | 1.26 to 14.48 | .012 |
| Histologic grade, 3 v 1 and 2 | 2.65 | 0.52 to 13.51 | .24 | 4.65 | 0.37 to 58.08 | .23 |
| Lymph node status | ||||||
| 1-3 v 0 | 2.37 | 0.49 to 11.41 | .28 | 7.86 | 1.21 to 51.08 | .031 |
| ≥ 4 v 0 | 10.77 | 4.34 to 26.75 | < .001 | 10.42 | 2.83 to 38.36 | < .001 |
| HER-2 status, positive v negative | 0.97 | 0.37 to 2.56 | .96 | 2.34 | 0.67 to 8.23 | .19 |
Abbreviations: ER-α66, estrogen receptor-α 66; DFS, disease-free survival; DSS, disease-specific survival; HR, hazard ratio; ER-α36, estrogen receptor-α 36; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
DISCUSSION
In the present study, we examined ER-α36 expression in specimens from 896 patients with breast tumor. We report here that patients with ER-α66–positive breast tumors that also express high levels of ER-α36 are less likely to benefit from tamoxifen treatment than patients who do not. In patients with ER-α66–positive breast tumors who received adjuvant tamoxifen treatment, ER-α36 expression was associated with poorer survival and remained as an independent unfavorable factor of DFS and DSS in multivariate analyses in this group. These findings are replicated in two independent cohorts. However, in patients with ER-α66–positive tumor who did not receive tamoxifen treatment but received chemotherapy alone, high levels of ER-α36 seemed to be a favorable factor for survival in a multivariate analysis, indicating that patients with both ER-α66– and ER-α36–positive tumors may be less likely to benefit from tamoxifen treatment but may benefit from chemotherapy. The notion that ER-α36 expression may predict the benefits of chemotherapy is supported in ER-α66–negative patients who received chemotherapy alone. Overexpression of ER-α36 tended to exhibit a favorable DSS in this subgroup in a multivariate analysis. Interestingly, a 21-gene assay has recently been developed and has been shown to accurately predict distant recurrence in patients with ER-α66–positive tumors treated with adjuvant tamoxifen.12 Patients with high recurrence score (RS) are less likely to benefit from tamoxifen treatment but gain a large chemotherapy benefit.13 Although our present findings may have potential clinical implications, they should not be translated into clinical practice until the data from prospective studies are available.
We also found that negative PgR and more than four lymph node metastases were independent unfavorable factors for survival in patients who only received tamoxifen treatment. These findings are concordant with previous studies that PgR status is an important determinant for tamoxifen treatment, patients with ER-α66–positive/PgR-positive tumors are more likely to benefit from tamoxifen treatment than those with ER-α66–positive/PgR-negative tumors.14,15 It is also well documented that four lymph node metastases is an unfavorable factor for patients who received tamoxifen treatment.15,16
In this study, we did not observe a correlation between ER-α66 and ER-α36 expression. Approximately 40% of ER-α66–positive or –negative tumors expressed high levels of ER-α36, suggesting that ER-α36 expression is independent of ER-α66 expression. The transcript of the ER-α36 isoform is initiated from a previously unidentified promoter in the first intron of the ER-α66 gene, suggesting that ER-α36 expression is regulated by a different promoter from ER-α66.8
HER-2 is a member of the epidermal growth factor receptor family. The HER-2 protein is overexpressed in 25% to 30% of breast cancers, where it is a marker of poor prognosis and an indicator of trastuzumab treatment.17–19 Laboratory and clinical evidence indicate that breast cancers that overexpress HER-2 are particularly less responsive to tamoxifen treatment.20–22 ER-α36 expression was associated with HER-2 expression in the first cohort of 710 consecutive patients; ER-α36 positive breast tumors more frequently expressed high levels of HER-2 compared with ER-α36–negative breast tumors. In agreement with this finding, our in vitro experiments demonstrated that block of HER-2 function using the HER-2–specific inhibitor AG825 in established breast cancer cells suppressed ER-α36 expression (Wang et al, unpublished data), suggesting that ER-α36 expression is positively regulated by the HER-2 signaling pathway. Thus it is possible that a signaling pathway mediated by HER-2 activates ER-α36 expression, which in turn confers tamoxifen resistance in HER-2–overexpressing tumors.
ER-α36 is predominantly located on the plasma membrane and in the cytoplasm and mediates membrane-initiated estrogen signaling.9 Membrane-initiated estrogen signaling has been linked to rapid responses to estrogen and generally activates signaling pathways like MAPK/ERK, phosphatidylinositol-3-kinase, and protein kinase C pathways.23–26 Thus it raises a possibility that ER-36 is a potential therapeutic target because tamoxifen cannot block ER-α36–mediated membrane-initiated estrogen signaling pathways.
In this study, we revealed that approximately 40% of ER-α66–positive breast cancer patients express high levels of ER-α36 in their tumors, and this subset of patients are less likely to benefit from tamoxifen treatment compared with those with ER-α66–positive/ER-α36–negative tumors. Although aromatase inhibitors are increasingly used for postmenopausal women with ER-α66–positive tumors,27 tamoxifen still remains as a first-line therapy for the foreseeable future, especially in premenopausal women in whom aromatase inhibitors are unlikely to be effective. Therefore, early identification of patients with ER-α66–positive breast cancer who may be resistant to tamoxifen treatment is extremely important in a clinical setting, because these patients may select an alternative endocrine therapy or other types of therapy from the diagnosis.
Appendix
Table A1.
Associations Between ER-α36 Expression, Clinicopathologic Characteristics, and Adjuvant Therapy in the First Cohort of 710 Patients
| Characteristic | No. of Patients | ER-α36 Expression |
P* | |||
|---|---|---|---|---|---|---|
| Positive |
Negative |
|||||
| No. | % | No. | % | |||
| Total | 710 | 280 | 39 | 430 | 61 | |
| Menopausal status | .006 | |||||
| Pre | 350 | 120 | 43 | 230 | 53 | |
| Post | 360 | 160 | 57 | 200 | 47 | |
| Tumor size, cm | .16 | |||||
| ≤ 2 | 441 | 165 | 59 | 276 | 64 | |
| > 2 | 269 | 115 | 41 | 154 | 36 | |
| Lymph node status | .89 | |||||
| 0 | 431 | 169 | 60 | 262 | 61 | |
| 1-3 | 146 | 60 | 22 | 86 | 20 | |
| ≥ 4 | 133 | 51 | 18 | 82 | 19 | |
| ER-α66 status | .73 | |||||
| Positive | 465 | 182 | 65 | 283 | 67 | |
| Negative | 237 | 96 | 35 | 141 | 33 | |
| Unknown | 8 | 2 | 6 | |||
| PgR status | .024 | |||||
| Positive | 355 | 126 | 45 | 229 | 54 | |
| Negative | 349 | 153 | 55 | 196 | 46 | |
| Unknown | 6 | 1 | 5 | |||
| HER-2 status | .005 | |||||
| Positive | 142 | 71 | 25 | 71 | 17 | |
| Negative | 564 | 209 | 75 | 355 | 83 | |
| Unknown | 4 | 4 | ||||
| Histologic grade | .72 | |||||
| 1 | 153 | 61 | 23 | 92 | 23 | |
| 2 | 469 | 192 | 70 | 277 | 70 | |
| 3 | 49 | 20 | 7 | 29 | 7 | |
| Unknown | 39 | 7 | 32 | |||
| Adjuvant therapy | .43 | |||||
| Chemotherapy | 280 | 113 | 42 | 167 | 40 | |
| Chemotherapy plus tamoxifen | 291 | 112 | 42 | 179 | 43 | |
| Tamoxifen alone | 91 | 39 | 14 | 52 | 13 | |
| No treatment | 24 | 6 | 2 | 18 | 4 | |
| Unknown | 24 | 10 | 14 | |||
Abbreviations: ER-α36, estrogen receptor-α 36; ER-α66, estrogen receptor-α 66; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
P values were derived from χ2 test.
Table A2.
Associations Between ER-α36 Expression and Clinicopathologic Characteristics in the Second Cohort of 186 Patients Who Only Received Tamoxifen
| Characteristic | No. of Patients | ER-α36 Expression |
P* | |||
|---|---|---|---|---|---|---|
| Positive |
Negative |
|||||
| No. | % | No. | % | |||
| Total | 186 | 86 | 46 | 100 | 54 | |
| Menopausal status | .62 | |||||
| Pre | 53 | 23 | 27 | 30 | 30 | |
| Post | 133 | 63 | 73 | 70 | 70 | |
| Tumor size, cm | .65 | |||||
| ≤ 2 | 120 | 54 | 63 | 66 | 66 | |
| > 2 | 66 | 32 | 37 | 34 | 34 | |
| Lymph node status | .039 | |||||
| 0 | 147 | 64 | 75 | 83 | 83 | |
| 1-3 | 20 | 8 | 9 | 12 | 12 | |
| ≥ 4 | 19 | 14 | 16 | 5 | 5 | |
| ER-α66 status | .90 | |||||
| Positive | 156 | 73 | 85 | 83 | 86 | |
| Negative | 27 | 13 | 15 | 14 | 14 | |
| Unknown | 3 | 3 | ||||
| PgR status | .61 | |||||
| Positive | 124 | 57 | 66 | 67 | 70 | |
| Negative | 58 | 29 | 34 | 29 | 30 | |
| Unknown | 4 | 4 | ||||
| HER-2 status | .40 | |||||
| Positive | 36 | 19 | 22 | 17 | 17 | |
| Negative | 149 | 67 | 80 | 82 | 83 | |
| Unknown | 1 | 1 | ||||
| Histologic grade | .60 | |||||
| 1 | 64 | 29 | 36 | 35 | 40 | |
| 2 | 99 | 48 | 59 | 51 | 58 | |
| 3 | 6 | 4 | 5 | 2 | 2 | |
| Unknown | 17 | 5 | 12 | |||
Abbreviations: ER-α36, estrogen receptor-α 36; ER-α66, estrogen receptor-α 66; PgR, progesterone receptor; HER-2, human epidermal growth factor receptor 2.
P values were derived from χ2 test.
Footnotes
Supported by the Program for New Century Excellent Talents in University (Grant No. 985-2-067-113 to Dr. Yuntao Xie) and a grant from the National Natural Science Foundation of China (Grant No. 30672419). This study was also supported by National Institutes of Health Grant No. DK070016 (Z.Y.W.), the Susan G. Komen Breast Cancer Foundation Grant No. BCTR81906 (Z.Y.W.), and the Nebraska Tobacco Settlement Biomedical Research Program Award (Grant No. LB-595; Z.Y.W.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Yuntao Xie
Financial support: Zhaoyi Wang, Yuntao Xie
Provision of study materials or patients: Tao Ouyang, Jinfeng Li, Tianfeng Wang, Zhaoqing Fan, Tie Fan, Benyao Lin, Yuntao Xie
Collection and assembly of data: Liang Shi, Yunwei Lu
Data analysis and interpretation: Liang Shi, Bin Dong, Zhongwu Li, Yuntao Xie
Manuscript writing: Zhaoyi Wang, Yuntao Xie
Final approval of manuscript: Liang Shi, Bin Dong, Zhongwu Li, Yunwei Lu, Tao Ouyang, Jinfeng Li, Tianfeng Wang, Zhaoqing Fan, Tie Fan, Benyao Lin, Zhaoyi Wang, Yuntao Xie
REFERENCES
- 1.Tamoxifen for early breast cancer. An overview of the randomised trials—Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 2.Jordan VC. Tamoxifen: A most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 3.Normanno N, Di Maio M, De Maio E, et al. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer. 2005;12:721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Bardou V, Hopp TA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK, Shou J, Massarweh S, et al. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–870s. [PubMed] [Google Scholar]
- 6.Gururaj AE, Rayala SK, Vadlamudi RK, et al. Novel mechanisms of resistance to endocrine therapy: Genomic and nongenomic considerations. Clin Cancer Res. 2006;12:1001s–1007s. doi: 10.1158/1078-0432.CCR-05-2110. [DOI] [PubMed] [Google Scholar]
- 7.Holm C, Rayala S, Jirstrom K, et al. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98:671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Zhang X, Shen P, et al. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhang X, Shen P, et al. A variant of estrogen receptor-{alpha}, hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorpe SM. Steroid receptors in breast cancer: Sources of inter-laboratory variation in dextran-charcoal assays. Breast Cancer Res Treat. 1987;9:175–189. doi: 10.1007/BF01806378. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Yao L, Li H, et al. Presence of erbB2 mRNA in the plasma of breast cancer patients is associated with circulating tumor cells and negative estrogen and progesterone receptor status. Breast Cancer Res Treat. 2006;97:49–55. doi: 10.1007/s10549-005-9086-7. [DOI] [PubMed] [Google Scholar]
- 12.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 14.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 15.Bardou VJ, Arpino G, Elledge RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 16.Fargeot P, Bonneterre J, Roche H, et al. Disease-free survival advantage of weekly epirubicin plus tamoxifen versus tamoxifen alone as adjuvant treatment of operable, node-positive, elderly breast cancer patients: 6-year follow-up results of the French adjuvant study group 08 trial. J Clin Oncol. 2004;22:4622–4630. doi: 10.1200/JCO.2004.02.145. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 18.Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 19.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 21.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 22.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer. 2001;8:191–195. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 23.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Björnström L, Sjoberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 25.Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades: Part 1. Membrane-associated signaling complexes. Trends Endocrinol Metab. 2002;13:349–354. doi: 10.1016/s1043-2760(02)00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzdar AU, Cuzick J. Anastrozole as an adjuvant endocrine treatment for postmenopausal patients with breast cancer: Emerging data. Clin Cancer Res. 2006;12:1037s–1048s. doi: 10.1158/1078-0432.CCR-05-2458. [DOI] [PubMed] [Google Scholar]