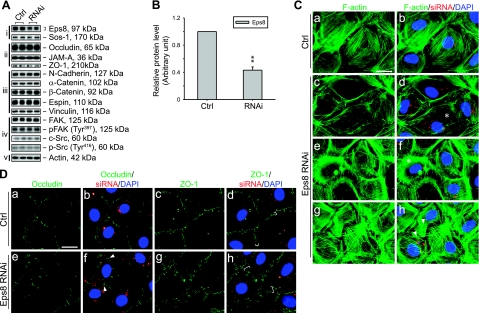
Figure 4.
Effects of Eps8 knockdown in primary Sertoli cell cultures in vitro. Eps8 was silenced by RNAi in primary cultured Sertoli cells with a functional TJ-permeability barrier mimicking the BTB in vivo. A) Effects of Eps8 silencing on steady-state protein levels related to junction dynamics: i) Eps8 and its partner Sos1; ii) TJ proteins; iii) basal ES proteins; iv) protein kinases known to regulate BTB dynamics; v) actin served as protein loading control. B) Histogram summarizing results shown in A after normalizing each data point against actin. Protein level in Ctrl was arbitrarily set as 1. Eps8 was knocked down by ∼60%, whereas no change in the steady-state levels in all other proteins was detected. Bars are means ± sd; n = 3. **P < 0.01; Student’s t test. C–D) Cells transfected with either Ctrl or Eps8 siRNA duplexes were tracked by cotransfection with siGLO red transfection indicator (red) and stained for F-actin, occludin, and ZO-1 (green). Nuclei were visualized by DAPI (blue). C) Changes in F-actin organization in SCs following Eps8 silencing. As compared to the uniformly distributed and orderly array of F-actin in control SCs (a, b), F-actin fibers in Eps8-silenced cells appear fragmented (c, d) and disorganized (e, f), as indicated by asterisks. In some Eps8-silenced cells (g, h), F-actin fibers appear overgrown and stretch over to neighboring cells (arrowheads). D) Redistribution and internalization of occludin and ZO-1 from the cell-cell interface resulting from Eps8 silencing. Both occludin and ZO-1 are localized primarily at the cell-cell interface in control cells (a–d). In Eps8-silenced cells (e–h), considerably more occludin appears to be redistributed and internalized, forming discrete aggregates in the cytosol (arrowheads), whereas ZO-1 staining appears diffused from the cell-cell interface (white bracket). Scale bars = 20 μm.