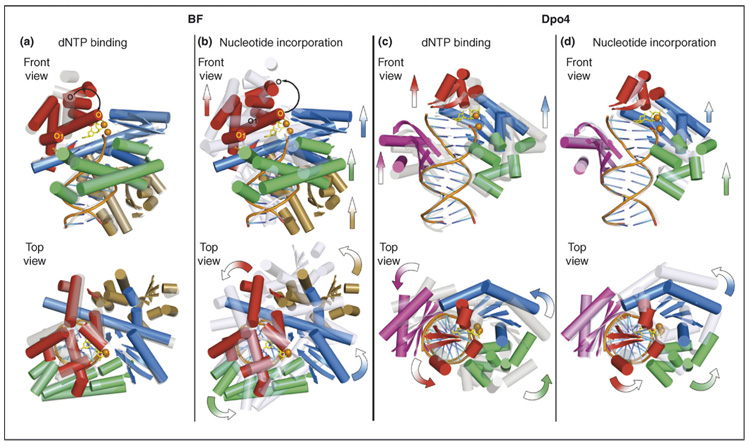
Figure 2.
Different translocation mechanisms for BF and Dpo4 suggested from their crystal structures. BF: a one-step translocation mechanism that takes place following the nucleotide-incorporation phase; Dpo4: a step-wise translocation mechanism that spans both dNTP-binding and nucleotide-incorporation phases. All structures are superimposed according to the backbone atoms in the DNA-duplex region. Ternary complexes are shown in a solid multicolor cartoon representation, and the short and long binary complexes are shown in transparent gray and light blue cartoon representations, respectively. For clarity, only α helices and β sheets of proteins and DNA backbone and base registers are shown. For ternary complexes, the incoming dCTP is shown in yellow sticks, and divalent metal ions are shown in orange spheres. The thumb domains are shown in green, palm domains in blue, fingers domains in red, the BF exonuclease domain in gold and the Dpo4 little finger domain in purple. The front views are into the major-groove side of the nascent base pair; the top views are in the 5′→3′ direction of the template strand. For the dNTP-binding phase, movements are directed from the transparent gray representation towards the solid multi-colored representation; for the dNTP incorporation phase, the movements are directed from the solid multi-colored representation towards the transparent light blue representation. Arrows in the front views denote translational movements, and curved arrows in the top views denote rotational movements. (a) Superimposition of the BF short binary (PDB code: 1L3T) and ternary (PDB code: 1LV5, chains A/C/D) complexes. A predominant protein conformational change is observed in the closing of the O helix (black curved arrow) in the fingers domain during the dNTP binding step, whereas no other marked translational or rotational movement of polymerase domains with respect to DNA is observed. (b) Superimposition of the BF ternary (PDB code: 1LV5, chains A/C/D) and long binary (PDB code: 1L3U) complexes showing the translocational movement of the thumb, palm and fingers domains upon dNTP incorporation. The O helix resumes its position in the binary complex (black curved arrow). (c) Superimposition of the Dpo4 short binary (PDB code: 2ASJ, chains A/D/E) and ternary (PDB code: 2ASD, chains A/D/ E) complexes showing the relocation of the polymerase domains during the dNTP-binding step, using the thumb–DNA contact points as the hinge. Note that no marked translational movement is observed for the thumb domain. (d) Superimposition of the Dpo4 ternary (PDB code: 2ASD, chains A/D/E) and long binary (PDB code: 2ASL, chains A/D/E) complexes showing the relocation of the polymerase domains during the dNTP incorporation step, using the little-finger–DNA contact points as the hinge. Note that no marked translational or rotational movement is observed for the little finger domain. In (c) and (d), the entire polymerase is relocated in two steps as a near rigid body, in which the overall protein conformation undergoes little change.