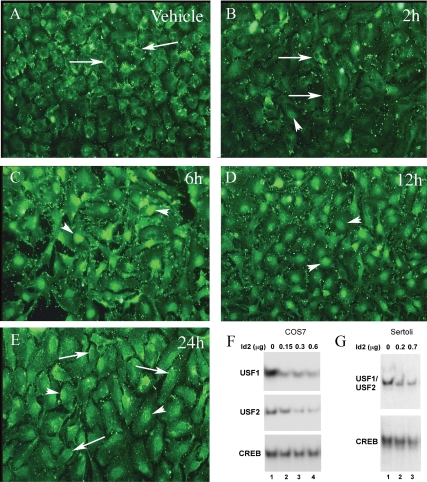
Figure 5.
FSH transiently increases ID2 levels in Sertoli cells and ID2 blocks USF protein binding to the Fshr promoter E-box. A–E, Cultured Sertoli cells were treated with vehicle or FSH (100 ng/ml) + IBMX (0.5 mm) for 2–24 h as indicated and probed with antiserum against ID2 (green). Arrows indicate perinuclear ID2 staining and nuclear exclusion of ID2. The arrowheads indicate nuclear staining of ID2. Results are representative of three independent experiments. Similar results were observed using two other ID2 antiserum (data not shown). F and G, EMSA studies were performed using either the Fshr E-box or consensus CREB binding site probe incubated with mixtures of whole-cell extracts from COS7 cells (F) or Sertoli cells (G) that were transfected with empty, USF, or ID2 expression plasmids. A constant level of extract (0.3 μg) from COS7 cells transfected with USF1 or USF2 expression plasmids were mixed with extracts from cells transfected with empty vector (lane 1) or 0.15, 0.3, or 0.6 μg of extract from cells transfected with ID2 expression vector. Extracts from cells transfected with empty vector were added to provide a total of 0.9 μg protein for each EMSA reaction (lanes 2–4). Binding of USF1 and USF2 to the Fshr E-box probe are shown in the top and middle panels, respectively. The binding of endogenous CREB proteins to the CREB probe is shown in the bottom panel. Whole-cell extracts from Sertoli cells transfected with either empty vector or ID2 expression vector were mixed and incubated with Fshr E-box or CREB probes in EMSA reactions. A total of 1.2 μg of extract was used for each DNA-protein binding reaction and consisted of 0, 0.2, or 0.7 μg from Sertoli cells transfected with ID2 expression vector. The relative binding of endogenous USF1/USF2 and CREB proteins to their respective probes are shown. The results displayed are representative of three (F) and two (G) independent experiments. Unbound probes were run off of the gels.