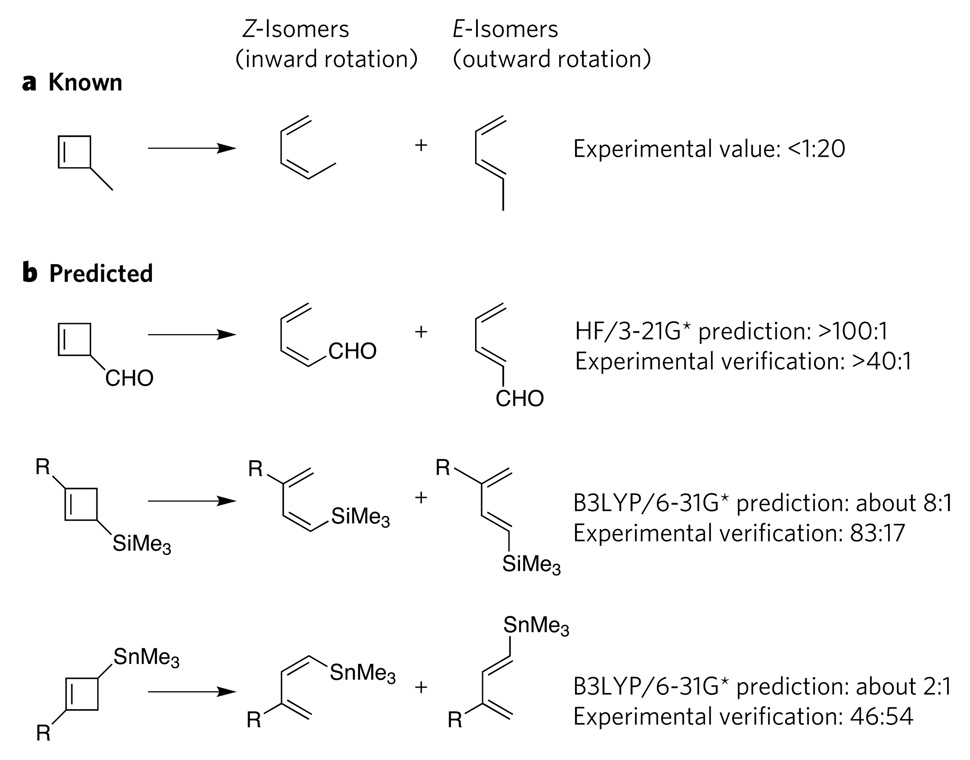
Figure 2. Successful computational predictions of non-catalytic reactions.
a, In cyclobutene ring-opening reactions, 3-methylcyclobutene forms mainly the E-isomer of 1-methylbutadiene (as well as a small amount of the Z-isomer); the experimentally determined ratio is <1 Z-isomer to 20 E-isomers. b, Using this knowledge as a starting point, computations correctly predicted the stereochemical outcomes of three analogous reactions5–7, as verified by subsequent experiments, even though the products were mainly Z-isomers rather than E-isomers. In these reactions, the electron-withdrawing substituents favour inward rotation, whereas, in the original reaction, the electron-donating substituent favours outward rotation. HF/3-21G* is an ab initio computational method, and B3LYP/6-31G* is a density-functional-theory computational method. Me, methyl; R, CMe2Ph.