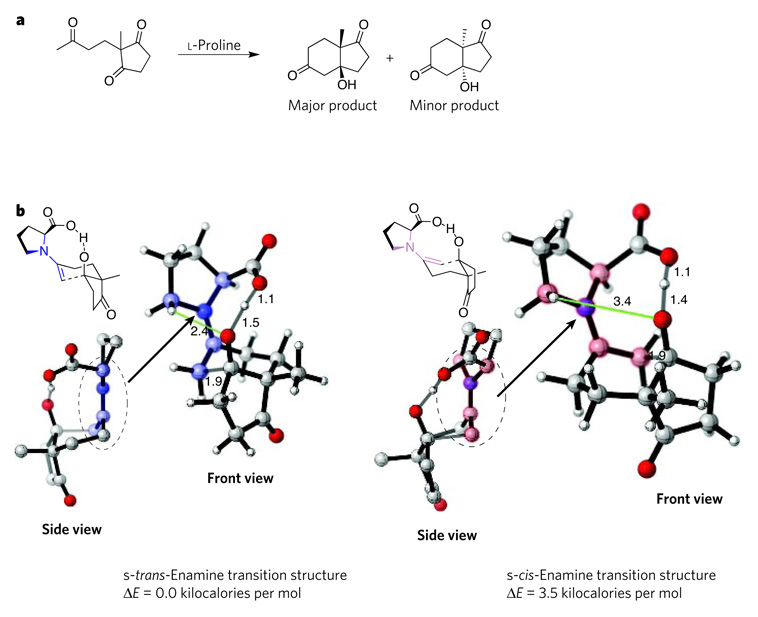
Figure 3. Transition structures of the Hajos–Parrish reaction.
The Hajos–Parrish reaction (a) was one of the first reported organocatalytic processes. Four possible transition states for this reaction were modelled12 using the hybrid density-functionaltheory method B3LYP and the basis set 6-31G*. The computations revealed that the most favoured transition state involves the s-trans-enamine intermediate (b, left), which incorporates a shorter, and therefore more strongly stabilizing, electrostatic interaction (green) between a hydrogen atom in the pyrrolidine ring and the oxygen atom of the developing alkoxide than does the s-cis-enamine intermediate (b, right). The side view of the s-trans enamine shows that this intermediate is a stable, undistorted (planar) developing iminium ion, whereas the s-cis-enamine intermediate is an unstable, distorted (non-planar) developing iminium ion. The favoured transition state leads to the formation of the major product that is observed experimentally, whereas the other transition state leads to the minor product. The main structural differences between the two intermediates are shaded in pale blue (s-trans form) and pale pink (s-cis form). Carbon is shown in grey, hydrogen in white, oxygen in red and nitrogen in blue. Distances between atoms are indicated in angstroms. Structures were generated using CYLview37. E, energy.