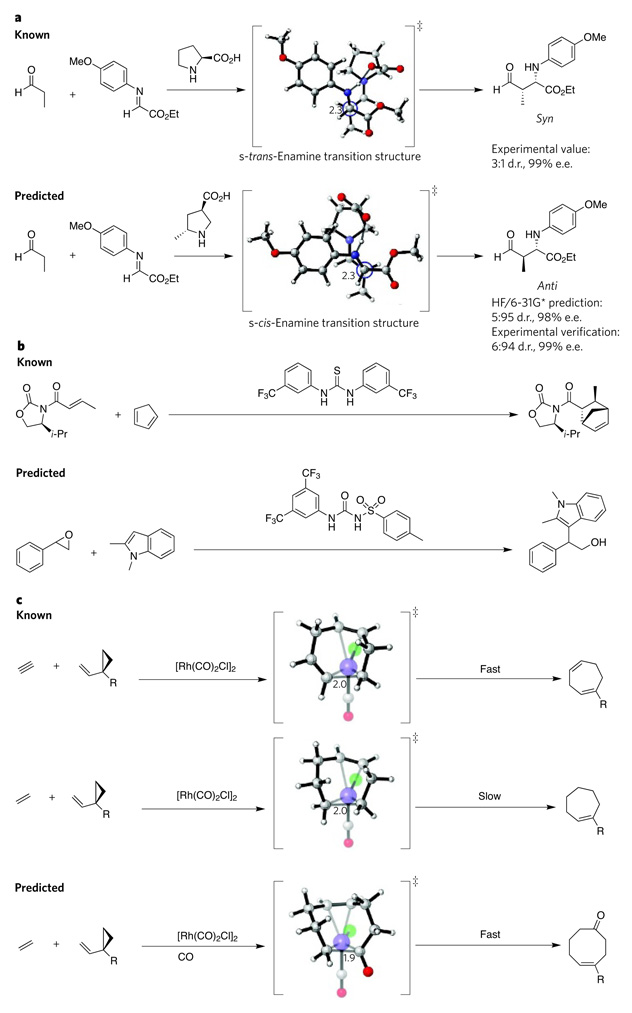
Figure 4. Successful computational predictions of catalysts.
a, Most Mannich reactions catalysed by organocatalysts mainly yield products in the syn configuration (top). Chemists designed an organocatalyst selective for the anti configuration (bottom) based on computational analysis of information from a proline-catalysed Mannich reaction16. b, The design of an epoxide-activating urea catalyst (bottom) came from the computational analysis of known thiourea catalysts (top) that activate carbonyl-containing compounds19. c, Rhodium-catalysed [5 + 2]-cycloaddition reactions of vinylcyclopropanes with allenes (not shown) or alkynes (top) are fast, but the analogous reactions with alkenes (centre) are slow. Computational analysis of these reactions led to the design of an analogous [5 + 2 + 1]-multicomponent-cycloaddition reaction that yields cyclooctenones23 (bottom). Carbon is shown in grey, hydrogen in white, oxygen in red, nitrogen in blue, chloride in green and rhodium in purple (large spheres). Critical carbon–carbon bond-forming distances in the transition structure are indicated in angstroms. Transition-state structures were generated using CYLview37. d.r., diastereomeric ratio; e.e., enantiomeric excess; Et, ethyl; i-Pr, isopropyl; R, alkoxy, alkyl, siloxy; ‡, transition state.