Figure 3.
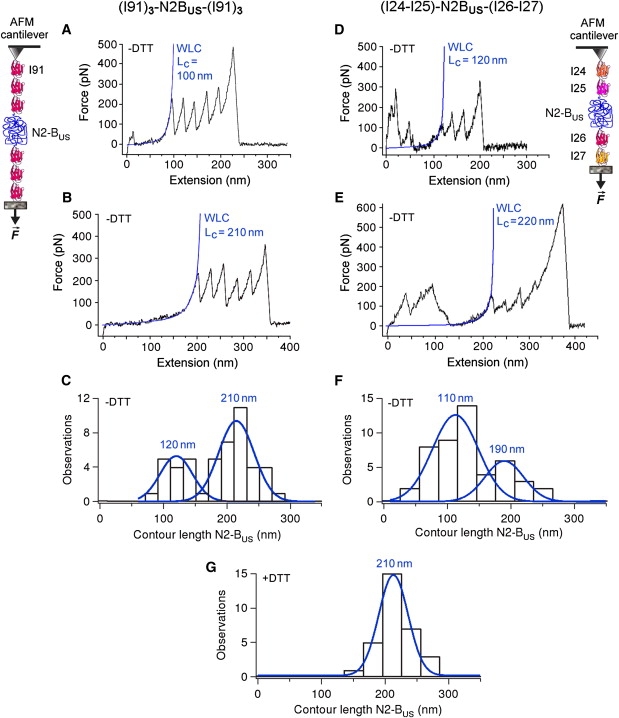
AFM force-extension experiments probing the presence of S-S bonds in the N2-Bus. Polyproteins (I91)3-N2-Bus-(I91)3 (left panels) and (I24)-(I25)-N2-Bus-(I26)-(I27) (right panels) were recombinantly expressed and stretched by AFM. Only those recordings that showed at least four regularly spaced Ig-unfolding peaks for (I91)3-N2-Bus-(I91)3 or three regularly spaced Ig-unfolding peaks for (I24)-(I25)-N2-Bus-(I26)-(I27) were analyzed by WLC fitting, because only then could we be confident that the whole N2-Bus had been stretched. (A–E) Exemplary recordings in 200 mM PBS buffer lacking reducing agent (−DTT), and WLC fit of N2-Bus force-extension behavior up to the first Ig-unfolding peak. Calculated contour length (Lc) values for the N2-Bus are indicated in blue text. (C and F) Histograms showing bimodal contour-length distribution in the absence of reducing agent (−DTT). Blue lines and values are single Gaussian fits and mean Lc. (G) Histogram of contour-length distribution for the N2-Bus in 200 mM PBS buffer supplemented with 10 mM DTT (+DTT). Blue line and value indicate single Gaussian fit and mean Lc. Note the absence of shorter contour lengths.
