Abstract
The plant-parasitic nematode Heterodera schachtii stimulates plant root cells to form syncytial feeding structures which synthesize all nutrients required for successful nematode development. Cellular re-arrangements and modified metabolism of the syncytia are accompanied by massive intra- and intercellular solute allocations. In this study the expression of all genes annotated as sugar transporters in the Arabidopsis Membrane Protein Library was investigated by Affymetrix gene chip analysis in young and fully developed syncytia compared with non-infected Arabidopsis thaliana roots. The expression of three highly up-regulated (STP12, MEX1, and GTP2) and three highly down-regulated genes (SFP1, STP7, and STP4) was analysed by quantitative RT-PCR (qRT-PCR). The most up-regulated gene (STP12) was chosen for further in-depth studies using in situ RT-PCR and a nematode development assay with a T-DNA insertion line revealing a significant reduction of male nematode development. The specific role of STP12 expression in syncytia of male juveniles compared with those of female juveniles was further shown by qRT-PCR. In order to provide evidence for sugar transporter activity across the plasma membrane of syncytia, fluorescence-labelled glucose was used and membrane potential recordings following the application of several sugars were performed. Analyses of soluble sugar pools revealed a highly specific composition in syncytia. The presented work demonstrates that sugar transporters are specifically expressed and active in syncytia, indicating a profound role in inter- and intracelluar transport processes.
Keywords: Electrophysiology, gene chip, Heterodera schachtii, in situ RT-PCR, sugar transporter, syncytium
Introduction
The obligate plant parasitic cyst nematode Heterodera schachtii infects roots of Arabidopsis thaliana in which it induces syncytial feeding cells (Sijmons et al., 1991). Unisex second-stage juveniles (J2) invade the roots and move intercellularly towards the central cylinder. There, they pierce one single cell with their stylet and release gland secretions which induce a dramatic re-organization of the tissue (Wyss, 1992). Local cell wall dissolution commences at plasmodesmata of the affected cells and leads to the formation of a syncytium consisting of up to several hundred cells (Grundler et al., 1998). Along with syncytium formation, the central vacuole fragments into several small vesicles, the nuclei enlarge, and cell organelles such as the smooth endoplasmatic reticulum, plastids, and mitochondria proliferate (Golinowski et al., 1996). Turgor pressure rises and osmotic potential drops in syncytial cells (Böckenhoff, 1995), reflecting high metabolic activity, elevated sucrose levels, and the formation of starch (Hofmann et al., 2007, 2008). The altered metabolisms of the affected root cells involve increased allocation of metabolites such as soluble sugars. Sugar transporters enable intra- and intercellular trafficking, ensure sugar retrieval, or act as direct sugar providers in connection with membrane-bound enzymes. Sugar import into syncytia also follows the symplasmic path during later stages of development, although most syncytia of early-stage females and males are symplasmically isolated (Hoth et al., 2005, 2008; Hofmann and Grundler, 2006; Hofmann et al., 2007). The different mechanisms of long- and short-distance transport towards and into nematode feeding sites have recently been reviewed (Hofmann and Grundler, 2007a). To date, there have been two studies on the expression of two sucrose transporters in nematode-induced syncytia (Juergensen et al., 2003; Hofmann et al., 2007). Given the 85 sugar transporter genes listed in the Arabidopsis Membrane Protein Library (AMPL www.cbs.umn.edu/arabidopsis/), these two represent only a small part of the picture. The large number of transporters reflects their different specific roles in physiology and development. In order to understand sugar transport mechanisms in syncytia it is therefore crucial to investigate the expression of a wide spectrum of sugar transporter genes in comparison with control roots. Affymetrix gene chip analysis is a valuable tool to study a large variety of expressed genes in various tissues. Such experiments have been performed for different plant–nematode interactions such as Meloidogyne incognita on Arabidopsis or tomato, and Heterodera glycines on soybean (Puthoff et al., 2003; Bar-Or et al., 2005; Hammes et al., 2005; Jammes et al., 2005; Ithal et al., 2007). Hammes et al. (2005) studied changes of transporter gene expression in Arabidopsis roots containing nematode galls induced by M. incognita. According to this study, sucrose and amino acid transporters as well as aquaporins are amongst the most highly regulated genes.
Data on the expression of sugar transporter genes give only a first hint of their possible role in cells. The actual activity is further determined by translation, protein turnover, allosteric control, competitive inhibition, and phosphorylation. Most sugar transporters operate by exploiting the proton-motive force. They are primarily energized by the activity of a proton pump (H+-ATPase) which carries protons outside a membrane and thereby generates a concentration difference of ∼100-fold. Movement of H+ through the plasma membrane can be measured as a transient depolarization.
Here a transcript analysis of all annotated sugar transporter genes expressed in syncytia induced by H. schachtii is presented. Six highly regulated sugar transporter genes were selected for quantitative RT-PCR (qRT-PCR) analysis and the most highly up-regulated gene was studied in detail. Electrophysiological recordings and transport studies with fluorochrome-labelled glucose confirmed the activity of sugar transporters in the syncytium plasma membrane. As a result of this combined molecular and physiological approach, it was demonstrated that transporters play a pivotal role in sugar transport into and within nematode-induced syncytia.
Materials and methods
Plant and nematode culture
Sterile Arabidopsis wild-type (Col) seeds were sown on 0.2% Knop medium and grown at 16 h light/8 h dark and 21 °C. Twelve days after germination plants were each inoculated with 50 freshly hatched second-stage juveniles (J2) (Sijmons et al., 1991) obtained from sterile stock culture.
RNA isolation and production of cDNA
Syncytia of 5-, 10-, and 15-d-old plants were excised from plant roots, each in three independent sampling events and immediately frozen in liquid nitrogen. As a reference, roots of non-infected plants omitting root tips were collected for each time point and treated in the same way. Total RNA was isolated using an RNase Plant Mini Kit (Qiagen, Hilden, Germany) according to the producer's instructions; including a DNase I digest (Qiagen). The quality of the RNA was checked using an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Reverse transcription was performed with the SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and random primers [oligo(dN)6] according to the manufacturer's instructions.
qRT-PCR
qRT-PCR was carried out with the ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA), and SYBR Green was used to detect transcripts. Primers were selected using Primer Express v2.0 Software (Applied Biosystems): At4g21480 for, tgtgccgttgtatctctcgg; rev, cgctagcctaaattgacctctctc; E=0.88; R2=0.990; At5g17520 for, ggtctttatctggatggacagcaac; rev, agccacatcaaatcacggataaata; E=0.83; R2=0.994; At1g61800 for, ctctgttcccggtcgctgta; rev: ccccataaacccagtgatgttg; E=0.83; R2 0.991; At5g27350 for, tgcaattggtgcactgttctg ; rev, catgttttggcgtaatttctgct; E=0.86; R2=0.992; At4g02050 for, cttcttgccggacggatca; rev, catgttcgctgtaaagatcccg; E=0.83; R2=0.993; At3g19930 for, tgcttctcattggtcgtatcctact; rev, cactgcagggacacaagctaatc; E=0.89; R2=0.995. Each qPCR sample contained 12.5 μl of Platinum SYBR Green qPCR SuperMix containing UDG and ROX (Invitrogen, Carlsbad, CA, USA), 3 mM MgCl2, 0.75 μl of forward and reverse primer (10 mM) for all genes except for At1g61800 for which 0.5 μl of primer (10 mM) was used, 2 μl of cDNA, and water to a total reaction volume of 25 μl. All samples were tested in triplicate; water was used as a control to rule out false-positive signals. Dissociation runs were performed to control for the possible formation of primer dimers. As internal references, 18S RNA and UBP22 were used which have been described to be stably expressed in syncytia (Hofmann and Grundler, 2007b). Samples were diluted 1:3, but 1:100 for 18S RNA. Results were obtained using the Sequence Detection Software SDS v2.0 (Applied Biosystems). Relative expression was calculated by the (1+E)-ΔΔCt method.
In situ RT-PCR
For in situ RT-PCR, root fragments were cut and put immediately into cold fixation solution as described in Koltai et al. (2001). After fixation, samples were embedded in 4% low melting agarose to make 20–30 μm cross-sections using a vibratome (VT 1000, Leica, Germany). RT-PCR was performed on the sections as described previously (Wieczorek et al., 2006). Cross-sections were examined under an inverted microscope (Axiovert 200M, Zeiss, Hallerbergmoos, Germany) with an integrated camera (AxioCam MRc5, Zeiss, Hallerbergmoos, Germany).
Affymetrix gene chip analysis
For gene chip analysis, pure syncytial cytosolic content was microaspirated at 5 d and 15 d after inoculation (dai) as described in Juergensen et al. (2003). As a control, root pieces cut from the elongation zone without root tips or lateral root primordia were used on the day of inoculation, representing time point zero. RNA extraction and sample preparation were performed as described previously (Wieczorek et al., 2006, 2008; Hofmann et al., 2008; Szakasits et al., 2009). ATH1 Arabidopsis gene chips (Affymetrix) were hybridized by RZPD (Deutsches Ressourcenzentrum für Genomforschung GmbH, Germany) according to the manufacturer's protocols. For the control roots and 5 dai syncytia, four biological replicates were used, and for 15 dai syncytia three biological replicates were used. Chip data are presented in Supplementary Table S1 available at JXB online; the complete data set has been published (Szakasits et al., 2009). Affymetrix.CEL files (from GCOS 1.2) were imported into the software program GeneSpring version 7.2 (Silicon Genetics, Agilent Technologies, Inc., Wilmington, DE, USA) using the RMA normalization pre-processor. Further calculation steps were performed as described in Hofmann et al. (2008).
Mutant screening
The T-DNA insertion mutant for At4g21480 (N518163) was from the Nottingham Arabidopsis Stock Centre (NASC; http://arabidopsis.info). Genomic DNA and total RNA were isolated from plants grown on MS medium containing 30 μg l−1 kanamycin. Gene-specific primers flanking the approximate site of T-DNA insertion and a gene-specific primer in combination with a T-DNA insertion-specific primer were used to analyse homozygocity and to verify the presence of the insertion as described on the NASC home page (http://signal.salk.edu/tdna_FAQs.html). RT-PCR was performed to show that the insertion prevents successful transcription. (At4g21480 for, gatggaaccccaggcgtttta; rev, tcaacgaacttcgaccaataccaatgt)
Nematode infection tests
Seeds of the T-DNA insertion line and the wild type (Col) were grown on Knop medium without supplemented sugar and with reduced nitrogen levels as described previously (Hofmann et al., 2007), and inoculated with nematodes. Root length was estimated at the date of inoculation as described in Jürgensen (2001). The experiment was repeated three times; every replicate consisted of five Petri dishes for each line. Two weeks after inoculation, female and male nematodes were counted; infection rates per cm of root length were calculated. Differences were calculated using the ANOVA-LSD test.
Carbohydrate analysis
Arabidopsis wild-type (Col) plants were grown on sand/soil (1:2 v/v) in 24-well plates. Each well contained ∼5–10 plants that were inoculated with ∼500 J2s after 12 d. Inoculated roots at 10 and 15 dai and control roots were washed and plant material was dissected as described. Soluble sugars from three independent sampling events, each consisting of 18–127 mg of fresh root material, were extracted with 60% ethanol for 30 min at 60 °C. After ethanol was evaporated in vacuo to dryness, sugars were dissolved in distilled water, diluted 4-fold, and analysed by HPLC–PAD (pulsed amperometric detection) on a Carbopac PA20 column (Dionex, Vienna, Austria) using a Dionex ICS3000 chromatography system. To separate galactose, glucose, and fructose, the column was thermostated at 30 °C and eluted with 10 mM NaOH at a flow rate of 0.5 ml min−1. Raffinose was eluted with 200 mM NaOH.
Phloem loading experiments
Plants were cultivated and inoculated with nematodes as described. At 3, 5, 7, 9, and 11 dai the phloem was loaded with 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxyglucose (2-NBD-glc) (Molecular Probes, Eugen, OR, USA) as described in Wright and Oparka (1997). Petri dishes were kept in darkness for 8 h. Fluorescence was monitored under an inverted microscope (Axiovert 200M, Zeiss, Hallerbergmoos, Germany) using UV light and a fluorescein isothiocyanate (FITC) filter.
Electrophysiological measurements
Syncytia containing root fragments (10–21 dai) were excised and placed in a groove of hardened, waterproofed two-component adhesive (UHU plus, UHU GmbH, Bühl, Germany), and soldered on at the cut ends with relatively low melting dental wax (Plano W. Plannet GmbH, Marburg/Germany). Periderm and other surrounding tissues were removed manually with glass knives made out of a 6 mm thick glass band (Plano W. Plannet GmbH, Marburg, Germany) with the LKB Bromma 7800 Knifemaker (Leica, Bernsheim, Germany). All preparations, except soldering, were done in bathing solution which contained 2.0 mM CaCl2, 1.0 mM MgCl2, 0.5 mM KCl, and 5.0 mM MES buffer adjusted to pH 6.4 with KOH.
Root parts were equilibrated for 1 h in the bathing solution containing 40 mM mannitol. Then mannitol was exchanged by glucose, fructose, sucrose, or raffinose (40 mM). To avoid artefacts this was done in random order of the sugars. Between sugar applications, samples were washed until the resting potential was stably recovered.
Microelectrodes consisted of borosilicate glass (capillaries with an outer/inner diameter of 1.0/0.58 mm) with a filament (GC100F-10, Clark Electrochemical Instruments, Pangbourne, Reading, UK), and were pulled on a horizontal puller Model P-2000 [(Sutter Instruments Co.) BioMetric System, Weiterstadt, Germany] as micropipettes with an outer tip diameter of 0.7 μm (resistance ∼106 Ω). They were placed in a MEH1SF microelectrode holder (World Precision Instruments, Berlin, Germany) which was mounted on the active measuring probe 712P (World Precision Instruments, Berlin, Germany). Microelectrodes and a home-made Ag/AgCl reference electrode (which connected the bathing solution with the ground) were filled with 0.5 M KCl. Signals were amplified by the Electrometer Duo773 (World Precision Instruments).
Microelectrodes were directed with a four-way micromanipulator MMO-202W (Narishige Co., Ltd, Tokyo, Japan) using 400-fold magnification (objective HC APO L 40×/0.80 W CORR, Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany).
Results
Gene expression of 85 sugar transporter genes annotated in the AMPL database was studied in nematode-induced syncytia using Affymetrix gene chips. Sample collection for such analyses is a critical step as nematode infection may affect neighbouring cells or provoke systemic effects. Therefore, the gene chips were hybridized with cDNA of root segments before infection (representing time point zero) and of microaspirated young-stage (5 dai) and late-stage (15 dai) syncytia (Szakasits et al., 2009).
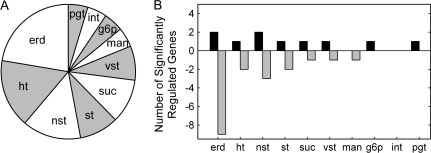
Sugar transporter genes were subdivided into 10 transporter families according to their putative function (cf. TAIR, http://www.arabidopsis.org) (see Fig. 1): erd (early responsive to dehydration stress), ht (hexose transporter), st (sugar transporter), suc (sucrose carrier), vst (vacuolar sugar transporter), man (mannitol transporter), nst (nucleotide sugar transporter), g6p (glucose-6-phosphate transporter), int (inositol transporter), and pgt (plastidial glucose transporter). The proportional occurrence of the single families in syncytia is indicated in Fig. 1A.
Fig. 1.
(A) Eighty-five putative sugar transporter genes were subdivided into 10 functional classes according to their putative function. Most are monosaccharide transporters as the information on di- and oligosaccharaide transporters is limited at present. erd (early responsive to dehydration stress), ht (hexose transporter), st (sugar transporter), suc (sucrose carrier), vst (vacuolar sugar transporter), man (mannitol transporter), nst (nucleotide sugar transporter), g6p (glucose-6-phosphate transporter), int (inositol transporter), and pgt (plastidal glucose transporter). (B) Significantly regulated genes in 15 dai syncytia analysed by Affymetrix gene chips. Each column presents the total number of significantly up- or down-regulated sugar transporter genes from single transporter families.
Affymetrix gene chip analysis of sugar transporter transcript levels
Gene chip analysis enabled differentiation of a number of highly expressed and significantly regulated genes. Most of the annotated genes are monosaccharide transporters, while the information on di- and oligosaccharide transporters is limited thus far. The number of significantly regulated genes of each sugar transporter family in 15 dai syncytia compared with the control at time point zero is presented in Fig. 1B (the complete data set is available as Supplementary Table S1 at JXB online; the entire chip data are published in Szakasits et al., 2009). Most of the significantly up- or down-regulated genes belong to the transporter families erd, ht, nst, and st. In detail, at 15 dai, 10 genes that belong to eight different sugar transporter protein families were significantly up-regulated (q <0.05) and 18 genes mostly belonging to the erd family were down-regulated (q <0.05) in syncytia compared with the control. The other down-regulated genes belonged to six different sugar transporter protein families (Fig. 1B). Comparing the two time points, only four genes (GPT2, ERD3, SUC1, and STP1) were differentially expressed (q >0.1) (see Supplementary Table S1). These data suggest that the expression of sugar transporters is adjusted specifically to syncytial conditions already in early stages, while fine-tuning may rely on post-transcriptional metabolic regulation at later stages.
qRT-PCR of highly up- and down-regulated candidate genes
The expression of the three highly up-regulated genes STP12, MEX1, and GPT2 and of the three highly down-regulated genes SFP1, STP7, and STP4 was further analysed by qRT-PCR in 5-, 10-, and 15-d-old syncytia compared with control roots (Table 1). In contrast to the gene chip analyses, the data are based on samples taken by cutting syncytia including surrounding tissue and non-infected roots of the same developmental stage. These data give a direct comparison of the relative changes of gene expression at an actual time point. The candidate genes have been chosen for further analyses since their significant expression pattern indicates an important role in sugar metabolism, or may unravel specifically down-regulated processes in syncytia (see Discussion).
Table 1.
Gene expression analysis of highly up- and down-regulated genes in syncytia with surrounding tissue in comparison with non-infected control roots by qRT-PCR (5, 10, and 15 dai)
| Locus | Gene | qRT-PCR | Chip | ||||||
| 5 dai | 10 dai | 15 dai | 5 dai | 15 dai | |||||
| Mean | SE | Mean | SE | Mean | SE | ||||
| At4g21480 | STP12 | 0.6 | 0.12 | 1.38 | 0.44 | –3.04 | 0.1 | 2.22 | 2.51 |
| At5g17520 | MEX1 | 1.24 | 0.30 | 1.993 | 0.43 | 1.68 | 0.55 | 1.68 | 1.71 |
| At1g61800 | GPT2 | 3.35 | 0.33 | 3.723 | 0.77 | 7.59 | 1.26 | 0.19 | 1.54 |
| At3g19930 | STP4 | –0.66 | 0.33 | –1.6 | 0.4 | –1.81 | 0.15 | 1.23 | –1.58 |
| At4g02050 | STP7 | –1.30 | 0.34 | –0.74 | 0.18 | –2.20 | 0.10 | –1.84 | –2.01 |
| At5g27350 | SFP1 | –1.05 | 0.53 | –1.51 | 0.17 | –2.56 | 0.39 | –3.41 | –3.49 |
Values are means ±SE, n=3 (log2). Gene chip results are based on microaspirated samples from syncytia at 5 dai and 15 dai and compared with root segments sampled at 0 dai (log2) (see Supplementary Table S1 at JXB online).
According to the results obtained by qRT-PCR, GPT2 was the most highly up-regulated gene at 5, 10, and 15 dai, followed by MEX1. STP12 was up-regulated at 5 dai and 10 dai, but down-regulated at 15 dai. SFP1, STP7, and STP4 were down-regulated at 5, 10, and, most strongly, at 15 dai. Even though the sampling for qRT-PCR differed from sampling for chip hybridization, the data correlated in most cases. Deviations were found in the expression of GPT2 which appeared to be more strongly regulated according to qRT-PCR, which may indicate a higher expression than that observed by the gene chip large-scale screening. The expression analysis of STP12 at 15 dai gave the only conflicting result of the two methods and was therefore analysed in more detail.
Expression and relevance of STP12 in nematode-induced syncytia
The most highly up-regulated gene identified by the gene chip analysis is STP12, indicating a specific role of this transporter for nematode-induced syncytia. The reduced values in this analysis therefore indicate a down-regulation of STP12 in the tissues surrounding the syncytia. To verify this conclusion, an additional set of microaspirated syncytia was collected and qRT-PCR was performed for STP12 (Fig. 2A), showing similar regulation patterns to those obtained by the gene chip.
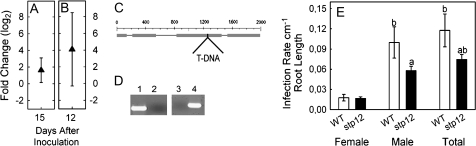
Fig. 2.
Detailed analyses of STP12 in nematode-induced syncytia. (A) qRT-PCR of microaspirated syncytia compared with control roots (15 dai) (values are means ±SE, n=3). (B) qRT-PCR of dissected syncytia of male juveniles compared with dissected syncytia of female juveniles (12 dai) (values are means ±SE, n=3). (C) The T-DNA insertion site in the exon of STP12. (D) PCR using T-DNA-specific primers of (1) the mutant line and (2) the wild type; RT-PCR using cDNA-specific primers of (3) the mutant line and (4) the wild type. (E) Nematode infection test comparing nematode development on Arabidopsis wild type (Col) plants with the T-DNA insertion lines of STP12. Letters indicate significant differences (P <0.05). Values are means ±SE, n=15.
Localization of STP12 transcripts by in situ RT-PCR was performed on cross-sections of syncytia and control roots. Amplified digoxigenin (DIG)-labelled transcripts are detected by specific antibodies conjugated to alkaline phosphatase that produces a purple stain in reaction with an NBT-BCIP (nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate) substrate. This method has previously been applied successfully for the localization of transcripts in root cross-sections (Wieczorek et al., 2006). Gene expression of STP12 was localized in syncytia at 15 dai (Fig. 3A). A slight background signal was obtained in syncytium cross-sections without polymerase treatment used as a negative control (Fig. 3B), whereas no signal occurred in cross-sections of non-infected control roots (Fig. 3C). These results are in agreement with the gene chip and the qRT-PCR analyses, and therefore confirm the observation of specific gene expression in syncytia.
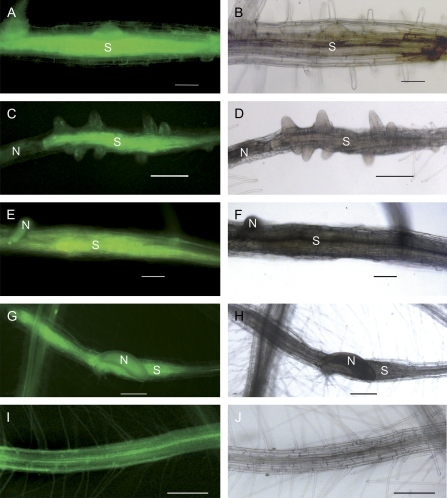
Fig. 3.
In situ RT-PCR on a cross-section showed STP12 expression (A) in syncytia, and (C) no expression in control roots at 15 dai. (B) As a negative control, polymerase was omitted. Bar=20 μm. S, syncytium.
The functional importance of the sugar transporter for nematode development was studied using an stp12 T-DNA insertion line obtained from the NASC (http://arabidopsis.info/). The T-DNA insert was located in the exon of the genes at position +1263 (Fig. 2C). T-DNA insertions were verified by PCR using insert-specific primers. Homozygosity of the mutants was tested by RT-PCR using gene-specific primers located behind the T-DNA insertion. If the mutant lines are homozygous, transcription is aborted so that no RT-PCR product is amplified (Fig. 2D). No phenotypic changes were evident in the mutant compared with the wild type. STP12 belongs to a large gene family, so that lack of expression may be compensated by homologous genes. The expression of STP12 may be induced under specific abiotic or biotic conditions, such as nematode infections, not affecting general plant growth.
The homozygous stp12 T-DNA insertion line was used in a nematode infection assay. Plants were grown on sugar-free medium in order to restrict the sugar supply to the syncytia and nematodes to naturally occurring transport mechanisms. Two weeks after inoculation plants were screened for the development of female and male nematodes and the infection rate per cm root length was calculated (raw data are shown in Supplementary Table S3 at JXB online). In the T-DNA insertion mutant line, the number of male nematodes was significantly reduced (Fig. 2E). These results suggest a higher relevance of STP12 for syncytia associated with male juveniles.
In order to prove this hypothesis, the expression of STP12 was analysed in syncytia of male juveniles in comparison with syncytia of female juveniles. For this experiment, sampling was performed at 12 dai because at this time point male and female juveniles can be differentiated unambiguously and most male juveniles are still actively feeding from their syncytia. As shown in Fig. 2B, the expression of STP12 is in fact higher in syncytia of male juveniles than in syncytia of female juveniles, supporting the presented concept.
Apoplasmic loading of glucose into syncytia
The experiments were based on the concept that syncytia of most of the early-tage nematodes are symplasmically isolated so that sugar import should only be possible via specific transporters. As most of the regulated transporter genes in syncytia are monosaccharide transporters (Fig. 1B), fluorochrome-labelled glucose was used (2-NBD-glc, see Material and methods) for in situ transport studies. 2-NBD-glc was previously used for uptake experiments into walled plant cells (Etxeberria et al., 2005). After application of 2-NBD-glc onto leaves of infected plants at 3, 5, 7, 9, and 11 dai, its phloem-mediated distribution to the roots (Fig. 4I) and uptake into syncytia could be traced (Fig. 4A–G). Thus, syncytia and attached nematodes were screened for fluorescent signals 8 h after loading. The fluorochrome was detected in almost all syncytia and nematodes already at 3 dai (Fig. 4A, Supplementary Table S2 at JXB online). In contrast, at this early stage of development no carboxyfluorescein diacetate (CFDA) was symplasmically translocated into syncytia (Hofmann et al., 2007). This result provides evidence that only sugar transporters are responsible for the import. For the other time points, the fluorochrome was also detected in almost all studied syncytia, showing a significantly different pattern from previous loading experiments with unspecific dyes (Hofmann and Grundler, 2006; Hofmann et al., 2007).
Fig. 4.
Phloem loading of 2-NBD-glc into Arabidopsis roots. Eight hours after loading, the fluorescent dye was detected in syncytia at (A) 3 dai, (C) 5 dai, (E) 7 dai, and (G) 9 dai, and (I) in non-infected control roots. (B), (D), (F), (H) and (J) show the same specimen as bright field images. Bar=0.2 mm. N, nematode; S, syncytium.
Electrophysiological measurements of the syncytial transmembrane potential
Activity of sugar transporters in the syncytial plasma membrane was verified by measuring the transmembrane potential upon application of different sugars using microelectrodes inserted into syncytia. Before insertion, root pieces were equilibrated in a mannitol-containing bathing solution to prevent osmotic effects. During the measurements, mannitol was exchanged by soluble sugars in a perfusion system. Each period of sugar application was followed by a mannitol wash until the resting potential had been recovered. Sugars were applied in succession to the same syncytia in order to obtain comparable data.
Typical depolarization/repolarization profiles in response to glucose, fructose, sucrose, or raffinose (40 mM) applied to 10–21 dai syncytia of female juveniles and adult females indicated active sugar transport (Fig. 5). Since male juveniles induce syncytia of much smaller size than female juveniles this analysis was technically restricted to syncytia associated with female animals. The most pronounced depolarization occurred after fructose and glucose application, which may indicate the highest transport potential for these sugars. While sucrose showed an intermediate reaction, depolarization after raffinose application was the lowest. The profiles suggest that phloem-derived sucrose and raffinose in the apoplast are imported as such or may be cleaved by the activity of cell wall-bound invertase and taken up by the syncytia as hexoses.
Fig. 5.
Typical changes in the syncytial transmembrane potential in response to application of different sugars. All applied sugars evoked changes typical for active uptake. After sugar application (0 s; all 40 mM) the potential depolarizes abruptly followed by a flattened depolarization and a repolarization. After removal of the sugars from the bathing solution (arrows), the membrane potential transiently exceeds the resting potential. Irregularities are due to so-called back shots of the plasma into the microelectrode generated by the high internal pressure of the syncytia.
Soluble sugar pools in syncytia
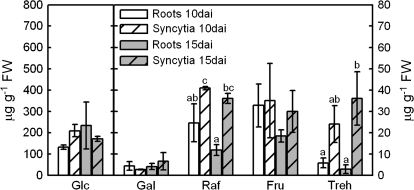
Given the fact that syncytia serve as the only source of nutrients to the nematodes, the composition of the sugar pool may be indicative of the conditions in the syncytial cells and the demands of the parasitizing nematodes. Pools of various soluble sugars were measured in syncytia and compared with control roots. Apart from sucrose (that was previously detected by Hofmann et al., 2007), glucose, galactose, raffinose, fructose, and trehalose were detected (Fig. 6). Glucose was the predominant sugar in syncytia and control roots at 10 dai and 15 dai. Levels of trehalose and raffinose were significantly higher in syncytia than in control root tissue, whereas glucose, galactose, and fructose were found to remain unchanged.
Fig. 6.
HPLC analysis of soluble sugars extracted from whole non-infected Arabidopsis roots and of excised nematode-induced syncytia (10 dai and 15 dai) of plants grown on sand/soil culture. Letters indicate significant differences (P <0.05). Values are means ±SE, n=3.
Discussion
Cyst nematodes have the ability to manipulate cell regulation and metabolism in order to adjust the highly specific conditions demanded for their successful development. Morphological re-arrangements and physiological changes are accompanied by mobilization of available resources. Here, evidence is provided that sugars are transported actively across the plasma membrane, but annotation data indicate further functions in intracellular transport.
In the present work, different approaches were followed in order to produce a consolidated line of evidence. In gene chip analyses, the expression of a broad range of transporter genes was monitored and highly specific expression patterns were found. The gene chip data used for this study have previously been validated for several groups of genes such as expansins, β-glucanases, genes coding for enzymes of the starch metabolic pathway, and others (Wieczorek et al., 2006, 2008; Hofmann et al., 2008; Szakasits et al., 2009). Several comparable transcriptome analyses have been performed in order to study plant–nematode interactions (Puthoff et al., 2003; Bar-Or et al., 2005; Hammes et al., 2005; Jammes et al., 2005; Ithal et al., 2007) and some of them include information about regulation of sugar transporter gene expression (Puthoff et al., 2003; Bar-Or et al., 2005; Hammes et al., 2005; Ithal et al., 2007). Arabidopsis roots infected by M. incognita, a root-knot nematode inducing separate hypertrophic giant cells embedded in hyperplastic gall tissue, expressed the sugar transporters SUC1, SFP1, and GPT1 as well as the vacuolar transporters VGT1 and TMT1 (Hammes et al., 2005). In nematode-induced syncytia, however, SUC1, GTP1, and VGT1 transcript levels were not regulated and SFP1 and TMT1 were significantly down-regulated. The most highly up-regulated gene in syncytia was that of the membrane transporter STP12 which is mainly expressed in flowers and seeds (Genevestigator: https://www.genevestigator.ethz.ch). It was the only considerably up-regulated gene coding for a transmembrane transporter. Further, the genes for two plastidial transporters (MEX1 and GTP2) were amongst the most highly up-regulated genes in syncytia indicating an induced starch metabolism as previously described (Hofmann et al., 2008). The results show that the most highly regulated sugar transporter gene in galls (SUC1) was not regulated in syncytia and none of the transporter genes highly regulated in syncytia was mentioned to be differentially expressed in galls (Hammes et al., 2005). Transcriptome analyses often show that several genes are up-regulated and others down-regulated within multigene families. This phenomenon was also found in the present work in the case of the ERD family. While some ERD genes were clearly down-regulated (At1g08930, At1g20450, and At3g20460), others (At2g48020, At4g19120, and At3g05400) were up-regulated. Individual genes of multigene families are often dedicated to specific functions in relation to developmental stages or plant organs, as may be the case here.
The expression levels of the three strongly up-regulated genes STP12, MEX1, and GTP2 and the strongly down-regulated genes SFP1, STP7, and STP4 (Table 1) were chosen to be tested by qRT-PCR. Analysing highly up- and down-regulated genes gives information about processes induced in syncytia and thus important for nematode parasitism and those processes that have a negative influence or have no specific role for nematode development and therefore become silenced. The first analysed gene is STP12 (At4g21480), a member of the ‘sugar transporter protein’ (STP) gene family. The transporter has high sequence homology with STP1 (Büttner and Sauer, 2000), a high-affinity glucose transporter in seedlings (Sherson et al., 2000) with substrate specificity for D-glucose, D-galactose, D-mannose, and D-fucose. STP12 appears to play the major role in hexose import into syncytia. MEX1 (At5g17520) is located in the plastidial membrane responsible for maltose export into the cytosol, emerging during starch degradation (Niittyla et al., 2004). Nematode-induced syncytia contain many plastids in which high levels of starch are stored (Hofmann et al., 2008). GPT2 (At1g61800) also codes for a putative plastidial translocator representing the main route for plastidial glc-6P/P import in non-photosynthetic active tissues. It has been described to be involved in sugar sensing as it is highly induced by application of sucrose and glucose (Gonzali et al., 2006). Further, GPT2 is induced in the starchless pgm mutant (Gonzali et al., 2006) and the SUC2-deficient pho3 mutant (Lloyd and Zakhleniuk, 2004) that are both characterized by increased levels of soluble sugars. The presence of plastid-specific transporters supports previous results that starch storage is employed for regulating sugar levels in syncytia. GTP2 was one of the few genes showing a significantly altered expression in 5 dai and 15 dai syncytia. Its induction at 15 dai indicates a higher starch metabolism requiring more glc-6P at that stage.
Amongst the strongest down-regulated genes was SFP1 (At5g27350), a senescence-related monosaccharide transporter (Quirino et al., 2001) responsible for sugar export. Syncytia are strong sink tissues in plants characterized by import processes so that the silencing of SFP1 is evident. The two strongest down-regulated hexose transporters were STP4 (At3g19930) and STP7 (At4g02050). Presently, there is no information about STP7 available, but STP4 has been characterized as sink specific and stress regulated (Truernit et al., 1996). Interestingly, STP4 was induced by wounding, bacterial elicitors, and pathogen challenge, and was suggested specifically to supply hexose to cover the increased energy demand of infection sites. The down-regulation of this transporter in syncytia suggests that nematodes do not induce general plant defence responses in syncytia.
While most results of the qRT-PCR confirmed the gene chip results, at 15 dai deviations between dissected and microaspirated syncytial samples were detected. STP12 transcript levels decreased in syncytium-enriched root samples, but the gene was up-regulated in microaspirated syncytial content hybridized onto gene chips as well as measured by qRT-PCR. Further in-depth studies were performed on the hexose transporter STP12 that was the most strongly up-regulated gene in the chip analysis. Transcripts of the transporter were specifically localized in syncytial cells by in situ RT-PCR (Fig. 3). A possible scenario may be that the transporter is down-regulated in the neighbouring cells in order to increase the efficiency of sugar uptake into syncytia. An additional line of evidence for the functional role of the transporter was created using a T-DNA insertion mutant. Transcription disruption led to a significant reduction of nematode development that affected males in particular. Most syncytia of male juveniles have previously been shown to remain isolated from the phloem (Hofmann and Grundler, 2006). Further, juveniles developing into males induce smaller syncytia in the pericycle (Sobczak et al., 1997). Probably this site of syncytium induction makes a symplasmic connection to the phloem improbable. Therefore, syncytia should be more dependent on intracellular sugar transporters than syncytia of female juveniles. In fact, this is reflected by increased transcript levels of STP12 in syncytia of male juveniles and their more sensitive response to the lack of STP12 expression in the mutant line. Thus additional evidence is provided that the organization of syncytia of males differs remarkably from that of females.
In order to show active sugar transport across the plasma membrane, phloem loading with the fluorescent 2-NBD-glc and electrophysiological measurements of transmembrane potentials were performed. Even though syncytia have been reported to be connected to phloem elements especially at later stages of development, this appears not to contradict the additional sugar import by transporters via the plasma membrane. Loading experiments with different types of labelled molecules result in different patterns of import into the syncytium. While experiments with CFDA indicate the symplasmic isolation of the majority of young syncytia, loading of 2NBD-glc gives evidence for the activity of sugar transporters in almost all young syncytia. Therefore, physiological analyses clearly demonstrate the activity of hexose transporters in the studied system that is closely related to transporter gene expression. Hexoses may primarily be imported by genes of the STP family. Next to STP12 that was the most highly up-regulated gene in syncytia, STP1 and STP3 could also play a role in hexose transport as both genes are expressed to a considerable extent in syncytia. In another plant–nematode interaction, the importance of glucose transporters for nematode-induced giant cells appeared to be minor. Jones et al. (1975) found no effect on the membrane potential of the plasma membrane of giant cells induced by M. incognita in roots of Impatiens balsamina in response to glucose application. Glucose was one of the predominant monosaccharides in syncytia; however, it was not accumulated significantly. Glucose underlies high turnover rates since it is the primary substrate for many polysaccharides such as starch that is formed in syncytia in order to balance high osmotic pressure (Hofmann et al., 2008). By performing electrophysiological analyses, sucrose and raffinose could also be identified as being imported into syncytia by transporter activity. According to transcript analyses, sucrose is primarily imported into syncytia by the products of SUC1, SUC2 and SUC4. The expression pattern of SUC2 and SUC4 in nematode-induced syncytia has been studied previously (Juergensen et al., 2003; Hofmann et al., 2007). Due to the moderate regulation of the sucrose transporters’ gene expression in syncytia, the physiological analyses suggest that their activity is regulated at a metabolic level. Sucrose was, after glucose, the dominant soluble sugar in syncytia (Hofmann et al., 2007). Its significantly increased levels indicate a specific role as an import sugar or as short-term storage, decreasing the osmotic potential of hexoses. Further, raffinose and trehalose levels were increased significantly in syncytia compared with control roots (Fig. 6). Both sugars have been described to be essential during drought stress or desiccation. Raffinose is the second major transport sugar in the phloem of Arabidopsis (Haritatos et al., 2000) but trehalose usually accumulates at rather low levels (≤0.15 μg g−1 fresh weight) in plants (reviewed in Schluepmann et al., 2004). Recently, much attention was focused on trehalose and its effects on plant growth, development, and stress resistance in view of its intriguing regulatory effects (reviewed in Rolland et al., 2006). Due to structural similarities to sucrose, trehalose was proposed to be an important device for sugar signalling (Ramon and Rolland, 2007). So far, no trehalose or raffinose transporter genes have been identified in Arabidopsis; however, several sugar transporters have been described with a high affinity for one substrate while transporting others with lower efficiencies (Gahrtz et al., 1994; Ludwig et al., 2000; Meyer et al., 2000; Sherson et al., 2000; Chandran et al., 2003). Therefore, raffinose and trehalose may be transported by less specific sucrose transporters. The measured sugars levels may also result from metabolic processing of carbohydrates.
In conclusion, the present data clearly support the hypothesis that sugar transporters play a pivotal role in the sugar transport and carbohydrate processing in nematode-induced syncytia in Arabidopsis and are therefore important for the development of the cyst nematode H. schachtii in this host.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Gene chip analysis of 5- and 15-d-old syncytia in comparison with non-infected root pieces. The normalized values show the transcript levels of the single genes in the specific tissues. Further, fold change (log2) of syncytia (syn) versus controls (con) and the two syncytia time points compared with each other are given. Asterisks indicate significances determined by a Benjamini–Hochberg multiple correction test (*q <10%; **q <5%; ***q <1%; see Materials and methods for details) (Benjamini and Hochberg, 1995).
Table S2. Arabidopsis phloem loading experiments with fluorescent labelled glucose. After 8 h the dye could be found in nematode-induced syncytia. Values are the total number (of three replicated experiments) of dye-positive and -negative syncytia as well as the total number.
Tab. S3. Raw numbers of root length and nematodes per Petri dish of the nematode infection assay (Fig. 2E).
Acknowledgments
The authors acknowledge the Austrian Science Fund for granting the project (project number P16897-B06 and P16296-B06) and the German Research Council (BE-1925/5-1).
References
- Bar-Or C, Kapulnik Y, Koltai H. A broad characterization of the transcriptional profile of the compatible tomato response to the plant parasitic root knot nematode Meloidogyne javanica. European Journal of Plant Pathology. 2005;111:181–192. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistic Society Series B. 1995;57:289–300. [Google Scholar]
- Böckenhoff A. Untersuchungen zur Physiologie der Nährstoffversorgung des Rübenzystennematoden Heterodera schachtii und der von ihm induzierten Nährzellen in Wurzeln von Arabidopsis thaliana unter Verwendung einer speziell adaptierten in situ Mikroinjektionstechnik. 1995. PhD thesis, University of Kiel, Germany. [Google Scholar]
- Büttner M, Sauer N. Monosaccharide transporters in plants: structure, function and physiology. Biochimica et Biophysica Acta. 2000;1465:263–274. doi: 10.1016/s0005-2736(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM. Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. Journal of Biological Chemistry. 2003;278:44320–44325. doi: 10.1074/jbc.M308490200. [DOI] [PubMed] [Google Scholar]
- Etxeberria E, González P, Tomlinson P, Pozueta-Romero J. Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. Journal of Experimental Botany. 2005;56:1905–1912. doi: 10.1093/jxb/eri185. [DOI] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N. A phloem-specific sucrose–H+ symporter from Plantago major L. supports the model of apoplast phloem loading. The Plant Journal. 1994;6:697–706. doi: 10.1046/j.1365-313x.1994.6050697.x. [DOI] [PubMed] [Google Scholar]
- Golinowski W, Grundler FMW, Sobczak M. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma. 1996;194:103–116. [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. Journal of Plant Research. 2006;119:115–123. doi: 10.1007/s10265-005-0251-1. [DOI] [PubMed] [Google Scholar]
- Grundler FMW, Sobczak M, Golinowski W. Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii. European Journal of Plant Pathology. 1998;104:545–551. [Google Scholar]
- Hammes UZ, Schachtman DP, Berg RH, Nielsen E, Koch W, M. McIntyre LM, Taylor CG. Nematode-induced changes of transporter gene expression in Arabidopsis roots. Molecular Plant-Microbe Interactions. 2005;18:1247–1257. doi: 10.1094/MPMI-18-1247. [DOI] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta. 2000;211:105–111. doi: 10.1007/s004250000268. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Grundler FMW. Females and males of root-parasitic cyst nematodes induce different symplasmic connections between their syncytial feeding cells and the phloem in Arabidopsis thaliana. Plant Physiology and Biochemistry. 2006;44:430–433. doi: 10.1016/j.plaphy.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Hofmann J, Grundler FMW. How do nematodes get their sweets: Solute supply to sedentary plant parasitic nematodes. Nematology. 2007a;9:451–458. [Google Scholar]
- Hofmann J, Grundler FMW. Identification of reference genes for qRT-PCR studies of gene expression in giant cells and syncytia induced in Arabidopsis thaliana by Meloidogyne incognita and Heterodera schachtii. Nematology. 2007b;9:317–323. [Google Scholar]
- Hofmann J, Szakasits D, Blöchl A, Sobczak M, Daxböck-Horvath S, Golinowski W, Bohlmann H, Grundler FMW. Starch serves as carbohydrate storage in nematode-induced syncytia. Plant Physiology. 2008;146:228–235. doi: 10.1104/pp.107.107367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann J, Wieczorek K, Blöchl A, Grundler FMW. Sucrose supply to nematode-induced syncytia depends on the apoplasmic and the symplasmic pathway. Journal of Experimental Botany. 2007;58:1591–1601. doi: 10.1093/jxb/erl285. [DOI] [PubMed] [Google Scholar]
- Hoth S, Schneidereit A, Lauterbach C, Scholz-Starke J, Sauer N. Nematode infection triggers the de novo formation of unloading phloem that allows macromolecular trafficking of green fluorescent protein into syncytia. Plant Physiology. 2005;138:383–392. doi: 10.1104/pp.104.058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Stadler R, Sauer N, Hammes ZU. Differential vascularization of nematode-induced feeding sites. Proceedings of the National Academy of Science, USA. 2008;105:12617–12622. doi: 10.1073/pnas.0803835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier R, Baum TJ, Mitchum MG. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Molecular Plant-Microbe Interactions. 2007;20:510–525. doi: 10.1094/MPMI-20-5-0510. [DOI] [PubMed] [Google Scholar]
- Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette M-L, Renou JP, Abad P, Favery B. Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. The Plant Journal. 2005;44:447–458. doi: 10.1111/j.1365-313X.2005.02532.x. [DOI] [PubMed] [Google Scholar]
- Jones MGK, Novacky A, Dropkin VH. Transmembrane potential of parenchyma cells and nematode-induced transfer cells. Protoplasma. 1975;85:15–37. [Google Scholar]
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, van Bel AJE, Grundler FMW. The companion cell-specific Arabidopsis disaccharide carrier AtSUC2 is expressed in nematode-induced syncytia. Plant Physiology. 2003;131:61–69. doi: 10.1104/pp.008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgensen K. Untersuchungen zum Assimilat- und Wassertransfer in der Interaktion zwischen Arabidopsis thaliana und Heterodera schachtii. 2001. Agrar- und Ernährungswissenschaftlichen Fakultät, Christian-Albrechts Universität, Kiel. [Google Scholar]
- Koltai H, Dhandaydham M, Opperman C, Thomas J, Bird D. Overlapping plant signal transduction pathways induced by a parasitic nematode and a rhizobial endosymbiont. Molecular Plant-Microbe Interactions. 2001;14:1168–1177. doi: 10.1094/MPMI.2001.14.10.1168. [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. Journal of Experimental Botany. 2004;55:1221–1230. doi: 10.1093/jxb/erh143. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Stolz J, Sauer N. Plant sucrose–H+ symporters mediate the transport of vitamin H. The Plant Journal. 2000;24:503–509. doi: 10.1046/j.1365-313x.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truenit E, Hümmer C, Besenbeck R, Stadler R, Sauer N. Atsuc3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. The Plant Journal. 2000;24:869–882. doi: 10.1046/j.1365-313x.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- Puthoff DP, Nettleton D, Rodermel SR, Baum TJ. Arabidopsis gene expression changes during cyst nematode parasitism revealed by statistical analysis of microarray expression profiles. The Plant Journal. 2003;33:911–921. doi: 10.1046/j.1365-313x.2003.01677.x. [DOI] [PubMed] [Google Scholar]
- Quirino BF, Reiter W-D, Amasino RD. One of two tandem Arabidopsis genes homologous to monosaccharide transporters is senescence-associated. Plant Molecular Biology. 2001;46:447–457. doi: 10.1023/a:1010639015959. [DOI] [PubMed] [Google Scholar]
- Ramon M, Rolland F. Plant development: introducing trehalose metabolism. Trends in Plant Science. 2007;12:185–188. doi: 10.1016/j.tplants.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signalling in plants: conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:576–675. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S. Trehalose mediated growth inhibition of arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiology. 2004;135:879–890. doi: 10.1104/pp.104.039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherson SM, Hemmann G, Wallace G, Forbes SM, Germain V, Stadler R, Bechtold N, Sauer N, Smith SM. Monosaccharide/proton symporter Atstp1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. The Plant Journal. 2000;24:849–857. doi: 10.1046/j.1365-313x.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, von Mende S, Burrows PR, Wyss U. Arabidopsis thaliana as a new model host for plant parasitic nematodes. The Plant Journal. 1991;1:245–254. [Google Scholar]
- Sobczak M, Golinowski W, Grundler FMW. Changes in the structure of Arabidopsis thaliana roots induced during development of males of the plant parasitic nematode Heterodera schachtii. European Journal of Plant Pathology. 1997;103:113–124. [Google Scholar]
- Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil D, Sykacek P, Grundler FMW, Bohlmann H. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. The Plant Journal. 2009;57:771–784. doi: 10.1111/j.1365-313X.2008.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. The Plant Cell. 1996;8:2169–2182. doi: 10.1105/tpc.8.12.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek K, Golecki B, Gerdes L, et al. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. The Plant Journal. 2006;48:98–112. doi: 10.1111/j.1365-313X.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- Wieczorek K, Hofmann J, Blöchl A, Szakasits D, Bohlmann H, Grundler FMW. Arabidopsis endo-1,4-β-glucanases are involved in the formation of root syncytia induced by Heterodera schachtii. The Plant Journal. 2008;53:336–351. doi: 10.1111/j.1365-313X.2007.03340.x. [DOI] [PubMed] [Google Scholar]
- Wright K, Oparka K. Metabolic inhibitors induce symplastic movement of solutes from the transport phloem of Arabidopsis roots. Journal of Experimental Botany. 1997;48:1807–1814. [Google Scholar]
- Wyss U. Observations of the feeding behaviour of Heterodera schachtii throughout development, including events during moulting. Fundamental and Applied Nematology. 1992;15:75–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.