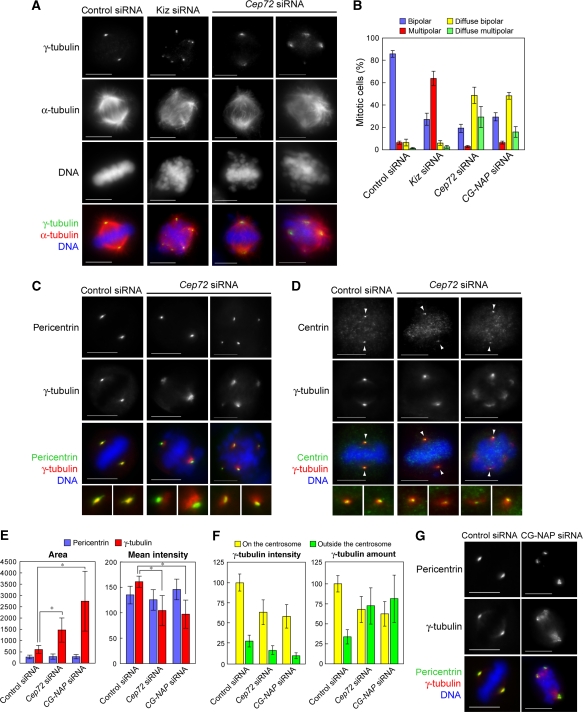
Figure 5.
Cep72 depletion causes spindle-pole fragmentation and diffuse γTuRC distribution around each spindle pole. (A) Control, Kiz, or Cep72 siRNA-transfected cells were fixed and immunostained for α-tubulin, γ-tubulin, and DNA. (B) The proportion of spindle phenotypes in control, Kiz, Cep72, or CG-NAP siRNA-transfected cells are categorized into four types: bipolar with focused (blue) or diffuse (yellow) γ-tubulin signals, and multi-polar with focused (red) or diffuse (green) γ-tubulin signals. Data represent the mean±s.d. of four experiments (n>100 for each experiment). (C, D) Control or Cep72-depleted HeLa cells were fixed 10–12 h after release from double thymidine block and immunostained for γ-tubulin and DNA, together with pericentrin (a marker for the PCM) (C) or centrin (a centriole marker) (D). The lower panels show magnified images of two spindle poles from each upper panel. Arrowheads indicate the centrioles. (E) Quantification of the area and intensity of pericentrin or γ-tubulin labelling at the poles in bipolar spindles in control, Cep72, and CG-NAP siRNA-treated cells. The stained areas were encircled by the edge of pericentrin or γ-tubulin staining and mean intensities of pericentrin and γ-tubulin in each area were measured. Data show the average intensity in the centrosomal area and represent the mean±s.d. (n>32). *P<0.001. (F) Quantification of the intensity of γ-tubulin signals inside and outside of the pericentrin-staining area. Total amount of γ-tubulin were results from multiplying the values of area and intensity. Data show the average intensities and amounts and the mean±s.d. (n=20). (G) Control or CG-NAP-depleted cells were immunostained for pericentrin, γ-tubulin, and DNA. Scale bar is 10 μm.