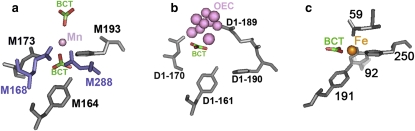
Figure 6.
Structures of the metal binding sites in (a) modified BRC, (b) PSII, and (c) in the C-lobe of ovotransferrin showing the key residues. The Mn in the BRC and the OEC in PSII are shown in pink, the iron in the ovotransferrin is orange, and the carbon and the oxygen atoms in the bicarbonate (BCT) are colored green and red, respectively. The mononuclear manganese in BRC in the M2 mutant is coordinated by His-M193, Glu-M173 and two genetically modified residues shown in blue: Glu-M168 and Asp-M288. The OEC is coordinated by several residues from the D1 subunit and from CP43. The residues from CP43 are not shown for simplicity. The corresponding residues of Glu-M173 His-M193 and Tyr-M164 of BRC are the Asp-D1-170 His-D1-190 and Tyr-D1-161. The mononuclear ferric ion in ovotransferrin is coordinated by Asp-59, Tyr-92, Tyr-191, His-250, and a BCT as a bidentate ligand. Coordinates for the M2 mutant with the proposed bicarbonates, PSII, and ovotransferrin are from Protein Data Bank entry codes 1Z9J, 1S5L, and 1OVT, respectively (12,37,38).