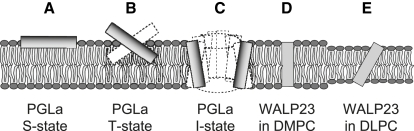
Figure 1.
Representative orientational states of membrane-bound peptides in the different peptide-lipid systems studied. (A) Surface-aligned amphiphilic peptide (S-state), PGLa/DMPC 1:200. (B) Tilted dimer (T-state), PGLa/DMPC 1:50. (C) Membrane-inserted oligomer (I-state) in a putative pore, PGLa/MAG/DMPC/DMPG 1:1:75:25. (D) Transmembrane state of a monomeric hydrophobic peptide, WALP23/DMPC 1:100. (E) Mismatched transmembrane state of a monomeric hydrophobic peptide, WALP23/DLPC 1:100. In all panels the hydrophobicity of peptides and lipids is indicated by a gray scale, where the more polar parts are shaded darker.