Figure 1.
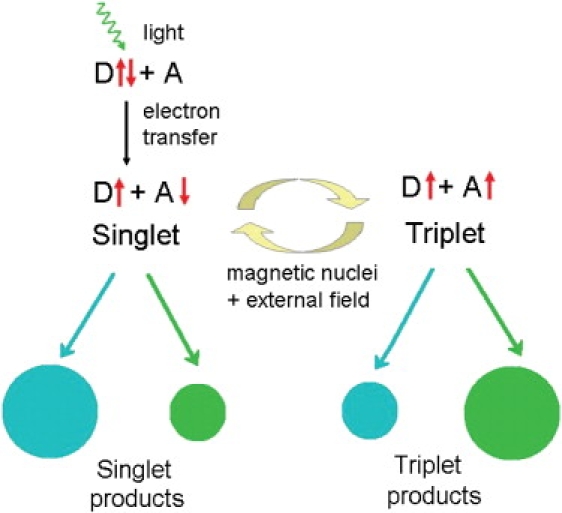
Schematic of the radical-pair mechanism. Light-induced electron transfer from a donor molecule D to an acceptor molecule A creates a radical pair, that is, two molecules each with an unpaired electron spin (up and down arrows next to D and A). Singlet and triplet states, defined by the relative orientation of the electron spins, interconvert due to the combined effects of internal and external magnetic fields. Singlet and triplet radical pairs decay into singlet and triplet products respectively, with relative yields indicated by the sizes of the circles. The relative yields of singlet and triplet products depend on the orientation of the external magnetic field with respect to that of the radicals. The arrows and circles at the bottom of the diagram symbolize pathways of product formation and reaction yields for two different orientations.
