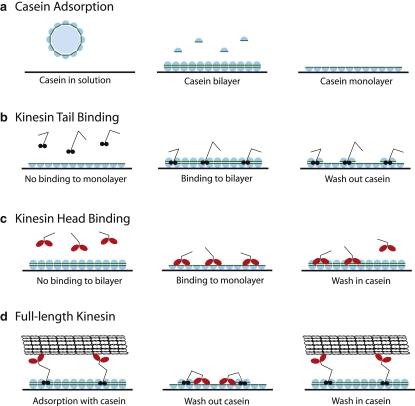
Figure 9.
Proposed mechanism for the interaction of casein, kinesin, and microtubules with SiO2 surfaces. (a) In solution, filtered casein exists as particles with mean diameter of 16 nm and, on interaction with the surface, dissociates into subunits that form a bilayer. Hydrophilic regions of the casein interact with the surface, and hydrophobic interactions stabilize interactions between the reversibly bound layer and the tightly bound layer. Removing the soluble casein results in dissociation of the reversibly bound casein, leaving a tightly bound monolayer on the surface. Note that the measured mass of the reversibly bound casein layer was only one-third of the tightly bound layer; however, for clarity here it is shown as a continuous layer. (b) In the absence of soluble casein, kinesin tails do not bind to the surface, but in the presence of soluble casein, the tails bind to the surface by interacting with both the tightly bound casein layer and the reversibly bound casein layer to form a tight interaction. Because of these stabilizing interactions, washing out soluble casein leaves both the tails and the associated subunits of reversibly bound casein on the surface. (c) In the presence of soluble casein, a casein bilayer blocks kinesin heads from interacting with the surface, but when soluble casein is removed, the heads bind to the tightly bound casein monolayer, presumably through hydrophobic interactions. This interaction is partially reversible, such that a wash step causes a portion of the heads to dissociate from the surface. (d) When adsorbed in the presence of soluble casein, kinesin binds to the surface through its tail domain, and the heads are free to interact with microtubules. Washing out the soluble casein results in the kinesin heads reversibly interacting with the tightly bound casein monolayer, such that reintroduction of soluble casein to form a bilayer rescues kinesin function.