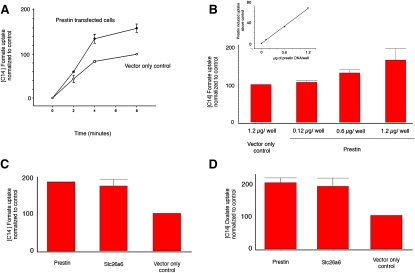
Figure 1.
Prestin-transfected CHO cells indicate anion transport. (A) CHO cells transfected with prestin show a time-dependent uptake of [14C]formate that was greater than that of CHO cells transfected with empty vector alone (control). Uptake of [14C]formate by prestin-transfected cells at 2, 4, and 8 min was 60 (±8.2), 135 (±10.4), and 160 (±9.5), respectively. In contrast, the uptake of control cells at corresponding time points was 44 (±8.2), 84 (±2.2) and 100 (n = 3). Data were normalized to vector-only controls at 8 min, which was given a value of 100. (B) There is a dose-dependent increase in [14C]formate uptake in cells transfected with increasing quantities of prestin-YFP plasmid. The mean uptake for cells transfected with 0.12 μg, 0.6 μg, and 1.2 μg of prestin plasmid DNA were 107 (±4), 131 (±8.5), and 166 (±31), respectively. Control wells were transfected with 1.2 μg of YFP plasmid DNA (n = 3). (Inset) Linear relationship between transport induced by prestin (after subtracting background) and amount of DNA used in transfection. (C and D) CHO cells transfected with prestin and Slc26a6 show increased uptake of [14C]formate (C) and [14C]oxalate (D) compared with control (YFP vector only). The plot shows mean uptake per 200,000 cells contained in a well (of a 24-well plate) ± SE. Data were normalized to vector-only controls (n = 3, in each case). Controls were assigned a value of 100. The uptake of [14C]formate by prestin and Slc26a6 was 183 (±0.7) and 171 (±17.3), respectively, i.e., significantly different from controls (p < 0.01, one-way ANOVA). The uptake of [14C]oxalate by prestin and Slc26a6 was 198 (±13.5) and 187 (±24.0), respectively, i.e., significantly different from vector-only controls (p < 0.05, one-way ANOVA). The absolute prestin-induced uptake of oxalate was 1.5-fold greater than formate uptake; this was obscured by normalization. Uptake is denoted in relative counts (see Methods).