Fig. 2.
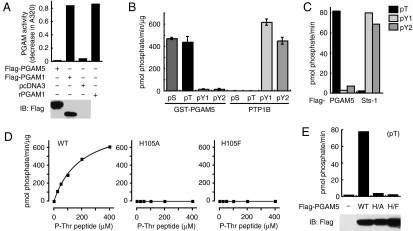
PGAM5 is a protein Ser/Thr phosphatase that activates ASK1. (A) PGAM5 lacks PGAM activity. Flag-PGAM5 (amino acids 29–289) lacking the TM domain, Flag-PGAM1, and pcDNA3 were individually expressed in HEK293 cells and were subjected to immunoprecipitation with anti-Flag antibody, followed by phosphoglycerate mutase assay in vitro. As a positive control, recombinant human PGAM1 (rPGAM1) was used instead of immunoprecipitated proteins. An aliquot of cell lysate was subjected to immunoblotting (IB). (B) PGAM5 specifically dephosphorylates phospho-Ser and -Thr peptides. Bacterially generated GST-tagged PGAM5 lacking the TM domain (GST-PGAM5) and PTP1B were subjected to in vitro phosphatase assay using phosphopeptides. Results shown are the means of triplicate determinations ± SD. (C) PGAM5 and Sts-1 exhibit strict substrate specificity. Flag-PGAM5 and Flag-Sts-1 were individually expressed in HEK293 cells and were subjected to immunoprecipitation with anti-Flag antibody, followed by in vitro phosphatase assay. (D) His-105 is essential for phosphatase activity of PGAM5. GST-PGAM5 with intact His-105 (WT), His105Ala, and His105Phe were subjected to in vitro phosphatase assay using various concentrations of phospho-Thr peptides. Results shown are the means of triplicate determinations. (E) PGAM5 immunoprecipitated from HEK293 cells exhibits His-105-dependent phosphatase activity. Flag-PGAM5-WT, -His105Ala (H/A), and -His105Phe (H/F) were individually expressed in HEK293 cells and were subjected to immunoprecipitation with anti-Flag antibody, followed by in vitro phosphatase assay. An aliquot of cell lysate was subjected to IB.