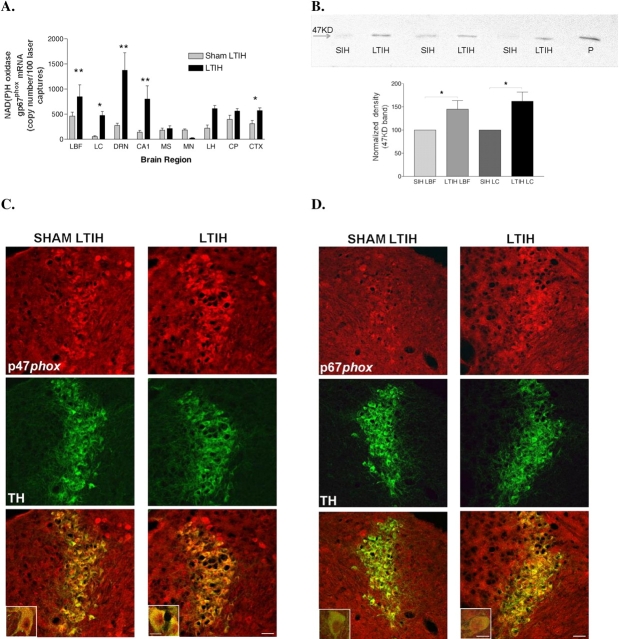
Figure 1.
Long-term intermittent hypoxia (LTIH), modeling oxygenation patterns in sleep apnea, results in increased NADPH oxidase gene and protein expression in wake-active brain regions. (A) NADPH oxidase subunit p67phox mRNA was measured in laser-captured neurons in brain regions selected because of behavioral state dependency or known hypoxia sensitivity. Taqman real-time reverse transcriptase polymerase chain reaction was performed on 50 laser-captured neurons each in the following brain regions: CA1 = hippocampal CA1 pyramidal cells; CTX = cortex; DRN = dorsal raphe nucleus; LBF = lateral basal forebrain (magnocellular preoptic, horizontal diagonal band, and substantia inominata); LC = locus coeruleus; LH = perifornicular lateral hypothalamus; MP = median preoptic area; MS = medial septum/vertical diagonal band; STR = striatum. Comparisons were drawn between wild-type mice exposed to LTIH (n = 10) and sham LTIH (n = 10). *p < 0.05; **p < 0.001. (B) NADPH oxidase sub-unit p47phox immunoreactivity (47 kD) in locus coeruleus and lateral basal forebrain micropunches in adult mice after LTIH (n = 8) and sham LTIH (n = 8) revealed increased p47phox in mice exposed to LTIH. *p < 0.01 in matched regions. P = positive control; SIH = sham intermittent hypoxia. (C, D) Immunohistochemical staining of noradrenergic locus coeruleus wake-active region shows enhanced NADPH oxidase subunit p47phox and p67phox expression (red) in locus coeruleus in mice exposed to LTIH. Double labeling with tyrosine hydroxylase localizes both subunits p47phox and p67phox to noradrenergic (tyrosine hydroxylase [TH], green) locus coeruleus wake-active neurons. Lower panel scale bar is 50 μm and inset bar is 20 μm.