Abstract
The formulation excipient Cremophor EL (CrEL) is known to limit absorption of oral paclitaxel given together with cyclosporin A (CsA). We hypothesized that the use of oral Genetaxyl, a paclitaxel formulation containing only 20% CrEL would have an improved oral bioavailability. Cohorts of 6 patients were treated with oral Genetaxyl at a dose of 60, 120, or 180 mg/m2 and 10 mg/kg of oral CsA in cycle 1. In cycle 2, patients received intravenous (i.v.) Genetaxyl (175 mg/m2, 3-hour infusion). Three additional patients received one dose of generic i.v. paclitaxel (Genaxol, containing 50% CrEL; 175 mg/m2, 3-hour infusion). The median area under the plasma concentration-time curve (AUC) and peak concentration of total paclitaxel following i.v. Genetaxyl were lower than those for i.v. Genaxol, as a result of significantly increased clearance (P = 0.017), and the AUC ratio for unbound to total paclitaxel for i.v. Genetaxyl was about 2 times higher than that for i.v. Genaxol (P = 0.0077). After oral administration of Genetaxyl at doses of 60, 120, and 180 mg/m2, the median total paclitaxel AUCs were 1.29, 1.60, and 1.85 µg×h/mL, respectively, suggesting a less than proportional increase in systemic exposure with increasing doses. The corresponding median values for the apparent bioavailability of oral Genetaxyl were similar when calculated either based on data for total paclitaxel (30.1%) or unbound paclitaxel (30.6%) when compared to i.v. Genetaxyl.
Keywords: Oral paclitaxel, Pharmacokinetics, Cyclosporin A, Formulation, Cremophor
Introduction
Paclitaxel is an anticancer agent widely applied in the treatment of advanced breast, lung and ovarian cancer [1–3]. However, intravenous (i.v.) administration of paclitaxel is inconvenient to patients and associated with significant and unpredictable side effects [4]. Theoretically, oral administration of paclitaxel is preferable because of its convenience, reduced administration costs, and potential use of more chronic treatment regimens. However, development of oral paclitaxel is hampered by poor bioavailability [5], which is due to extensive first-pass metabolism by the cytochrome P450 isoforms CYP2C8 and CYP3A4 [6] and, to a lesser extent, to paclitaxel’s affinity for efflux transporters expressed in the gastrointestinal tract and liver, such as ABCB1 (P-glycoprotein) [5] and ABCC2 (cMOAT; MRP2) [7]. Strategies used to improve the oral bioavailability of paclitaxel include co-administration of enzyme/transporter inhibitors such as cyclosporin A (CsA) with paclitaxel, and, more recently, the development of alternative formulations of paclitaxel on the basis of mixed micelles, nanoemulsions, or lipid nanocapsules [8–12].
CsA is an efficacious inhibitor of CYP3A4 [13], ABCB1 [14], and ABCC2 [15], and the feasibility of co-administering CsA with oral paclitaxel to increase systemic exposure has been demonstrated in various Phase 1 [16–18] and Phase II clinical trials [19,20]. However, the apparent bioavailability of oral paclitaxel is still relatively low (about 30%), which is partly due to the presence of high concentrations (50%, v/v) of the formulation excipient Cremophor EL (CrEL), which limits drug absorption through the formation of micelles that entrap paclitaxel [21]. Furthermore, the high content of CrEL poses issues related to the calculation of bioavailability because its presence in the conventional paclitaxel formulation is associated with nonlinear drug disposition after i.v. administration [22]. In theory, paclitaxel formulated in solvents with less or no CrEL when given orally together with CsA may be associated with an improved oral bioavailability. Here, we investigated the pharmacokinetics of a novel paclitaxel formulation (Genetaxyl) containing only 20% (v/v) CrEL [23] given orally in combination with CsA in cancer patients.
Materials and Methods
Patient selection criteria
Patients over 18 years old with a histologically or cytologically proven malignancy for which no standard therapy of proven benefit was available were eligible for the study. Tumor types were selected based on hypothetical benefit from paclitaxel treatment according to the published data, and included head and neck cancer, gastric cancer, esophageal cancer, and urinary bladder cancer. Previous radiotherapy and/or chemotherapy other than taxane-based therapy was allowed as long as the last treatment was at least 4 weeks prior to study entry and any remaining toxicities had resolved. Patients had to have acceptable bone marrow function (hemoglobin ≥ 9 g/dL; white blood cell count ≥ 3.0 × 109 cells/L; absolute neutrophil count ≥ 1.5 × 109 cells/L; platelet count ≥ 100 × 109 cells/L), liver function (total serum bilirubin ≤ 1.5 × the upper limit of normal; serum transaminases ≤ 3 × the upper limit of normal in the absence of liver metastases and ≤ 5 × the upper limit of normal in the presence of liver metastases), and kidney function (serum creatinine ≤ 1.5 × the upper limit of normal), and a Eastern Cooperative Oncology Group performance status ≤ 2.
Patients were excluded if they had a serious medical or psychiatric illness that would prevent giving informed consent and in case of a life expectancy less than 3 months. Patients were also excluded if they suffered from uncontrolled infectious disease, neurologic disease with greater than grade 1 motor/sensory neurotoxicity from previous treatment or diseases, bowel obstruction or impaired swallowing function (such as tube feeding), uncontrolled or severe cardiovascular disease, or symptomatic brain metastases. Females of childbearing potential were required to have a negative serum pregnancy test prior to study entry. Further exclusion criteria included known or suspected hypersensitivity to CrEL, polyethyeneglycol 300, polysorbate 80 (Tween 80), ethanol, or CsA and concomitant use of known ABCB1 inhibitors and chronic use of H2-receptor antagonists or proton pump inhibitors. The study protocol was approved by the Medical Ethics Committee of the Institute, and all patients signed written informed consent.
Paclitaxel administration
The Genetaxyl preparation was composed of paclitaxel formulated in 20% (v/v) absolute ethanol, 20% (v/v) CrEL, polyethyleneglycol 300, and a small (<11% (v/v)) amount of polysorbate 80 [23]. Genaxol, a generic formulation of Taxol, was composed of paclitaxel formulated in absolute ethanol and CrEL at a 1:1 (v/v) ratio. Both products were developed by Genovate Biotechnology (Hsinchu, Taiwan) and were supplied as a clear sterile solution in 5 mL-vials containing 30 mg of paclitaxel (concentration of paclitaxel, 6 mg/mL). For oral administration, Genetaxyl was diluted in 5% dextrose water solution prior to administration. Doses of Genetaxyl of 60, 120, or 180 mg/m2 were dissolved in about 45, 65, or 85 ml of 5% dextrose solution (final paclitaxel concentrations, ∼2, ∼3, and ∼3.5 mg/ml), respectively. CsA (Neoral, Novartis, Basel, Switzerland) was used as a modulator of paclitaxel pharmacokinetics because the existence of prior data obtained with this agent allowed for a preliminary comparison of data obtained with Genetaxyl compared with Taxol [17]. CsA was administered orally at a dose of 10 mg/kg to patients 30 minutes before oral intake of Genetaxyl. The dosages were selected based on prior data suggesting that systemic paclitaxel exposure did not increase as the oral dose was increased from 180 to 540 mg/m2 [17], and that systemic exposure to paclitaxel was not further affected by doses of CsA above 10 mg/kg [24]. For i.v. administration, Genetaxyl or Genaxol preparations were diluted in 500 mL of 5% dextrose or 0.9% normal saline in non-polyvinylchloride-containing containers with micropore filters. Infusion pumps were used for precise control of the infusion rates.
Three patients were planned to receive one dose of i.v. Genaxol with pharmacokinetic sampling as a 3-hour infusion at a dose of 175 mg/m2 on day one of a three-weekly cycle (control group). These patients could receive subsequent cycles of treatment with either i.v. Genetaxyl or i.v. Genaxol for up to 5 cycles, if clinically indicated. Eighteen patients, 6 per dose level, were planned to receive one dose of oral Genetaxyl. Subsequently, patients were scheduled to receive one dose of i.v. Genetaxyl on day one of cycle 2, as a 3-hour infusion at a dose of 175 mg/m2. They were eligible to receive an additional 4 cycles of the same regimen, if clinically indicated. Toxicities were recorded for safety evaluation according to the National Cancer Institute-Common Toxicity Criteria version 2.0.
Premedication and CsA administration
Premedication for cycle one included 20 mg of i.v. dexamethasone, 5 mg of i.v. tropisetron (both given about 1 hour before Genetaxyl), 50 mg of i.v. diphenhydramine, and 50 mg of i.v. ranitidine (both given about 30 minutes before Genetaxyl). For i.v. Genetaxyl or i.v. Genaxol, premedication included 20 mg of i.v. dexamethasone (about 1 hour before), 50 mg of i.v. diphenhydramine, and 50 mg of i.v. ranitidine (about 30 minutes before). Standard antiemetic therapy was allowed if needed.
Blood sampling and processing
Blood sampling for pharmacokinetic analysis was performed for each of the three different administrations (oral Genetaxyl, i.v. Genetaxyl, and i.v. Genaxol) after the first dose. Blood samples were collected from a peripheral site contralateral to the venous access used for drug infusion. For i.v. Genetaxyl and Genaxol, samples were obtained before drug administration, 1.5 hours after the start of the infusion, 2 minutes before the end of infusion, and 6, 18, 30, and 60 minutes and 2, 4, 8, 12, 24, 30, and 48 hours after the end of the infusion. For oral Genetaxyl, blood samples were obtained immediately before treatment, and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 7, 10, 24, 30, 48, and 56 hours after drug administration. All blood samples were centrifuged at 2000 × g for 15 minutes at 4 °C to separate plasma, which was stored at or below –70 °C until the time of analysis.
Analysis of total paclitaxel concentrations
Concentrations of total paclitaxel in plasma samples were determined by a validated method based on high-performance liquid chromatography with tandem mass-spectrometric detection [25]. The lower limit of quantitation of this method is 2 ng/mL, and the range for calibrators was 2 to 2,500 ng/mL. The percent deviation from nominal values in quality control samples was less than 8%, and the within-run and between-run precision was less than 15%.
Analysis of unbound paclitaxel concentrations
The unbound concentration of paclitaxel in patient samples was determined using a validated method based on micro-equilibrium dialysis [26]. The fraction unbound paclitaxel was calculated after subtracting the background radioactivity reading, and unbound concentrations were calculated as the product of the total plasma concentration and the fraction unbound paclitaxel.
Pharmacokinetic analysis
Estimates of pharmacokinetic parameters for paclitaxel were derived from individual concentration-time data sets using non-compartmental methods implemented in the computer software program WinNonlin v4.0 (Pharsight Corporation, Mountain View, CA). The peak plasma concentration (Cmax) was the observed value. The area under the plasma concentration-time curve (AUC) from time zero to infinity was calculated using the log-linear trapezoidal method by extrapolation to infinity by dividing the last measured concentration by the terminal rate constant, λz, which was determined from the slope of the terminal phase of the concentration-time curve using weighted least-squares as the estimation procedure, and inverse variance of the output error (linear) as the weighting option. The mean (± standard deviation) percent extrapolation of the AUC beyond the last sampling time point was 9.64 ± 3.68%. The terminal half-life (t1/2) was calculated as 0.693 divided by λz. Additional pharmacokinetic parameters included the volume of distribution at steady-state (Vss) and the systemic clearance, which was calculated as dose divided by AUC. The time to peak concentration (Tmax) was obtained from the experimental data. The apparent oral bioavailability of total and unbound paclitaxel was calculated as the dose-corrected ratio of median AUC values after oral Genetaxyl administration and i.v. Genetaxyl administration.
Statistical evaluation
All pharmacokinetic parameters are reported as median with range, unless stated otherwise. The effect of the respective formulations of Genaxol and Genetaxyl on the systemic clearance of paclitaxel was evaluated using a nonparametric Mann-Whitney U test. The effect of drug dose on the apparent oral bioavailability and apparent oral clearance obtained after oral administration was assessed using a nonparametric Kruskal-Wallis test. Statistical calculations were performed using the software program Number Cruncher Statistical Systems (version 2001; NCSS, Kaysville, UT). The a priori level of statistical significance was set at 0.05.
Results
Patient characteristics
A total of 23 patients enrolled on this study (Table 1). Two patients were not assessable because they went off study due to violation of inclusion or exclusion criteria after they had received only oral Genetaxyl in cycle one. In total 18 patients (6 at each dose level) were treated at each consecutive Genetaxyl dose level of 60, 120 and 180 mg/m2, and complete pharmacokinetic profiles were evaluable on all patients. These same 18 patients were treated in cycle 2 with a 3-hour i.v. infusion of Genetaxyl at a dose of 175 mg/m2. Of these, two patients had extremely high paclitaxel concentration values measured in the samples drawn 1.5 hours after the start of infusion, suggesting that the sample was not drawn from a site away from the site of infusion, and were excluded entirely. Another patient was not assessable because blood samples could not be obtained from a peripheral site due to a clotted catheter. Eventually, complete data were obtained from 15 patients for the i.v. Genetaxyl analysis. For comparative purposes, three additional patients received one dose of Genaxol (generic Taxol) as a 3-hour i.v. infusion at a dose of 175 mg/m2.
Table 1.
Patient demongraphicsa
| Characteristics | Genaxol (n=3) | Genetaxyl (n=20) | All patients (n=23) |
|---|---|---|---|
| Age (years) | |||
| Median | 53.2 | 54.6 | 54.5 |
| Range | 41.6–55.0 | 30.6–76.6 | 30.6–76.6 |
| Sex | |||
| Male | 3 (100%) | 19 (95%) | 22 (96%) |
| Female | 0 | 1 (5%) | 1 (4%) |
| Performance status | |||
| 0 | 1 (33%) | 2 (10%) | 3 (13%) |
| 1 | 0 (0%) | 18 (90%) | 18 (78%) |
| 2 | 2 (66%) | 0 | 2 (9%) |
| Body surface area (m2) | |||
| Median | 1.38 | 1.66 | 1.65 |
| Range | 1.20–1.54 | 1.50–1.94 | 1.20–1.94 |
| Primary tumor type | |||
| Stomach | 1 (33%) | 2 (10%) | 3 (13%) |
| Head & Neck | 2 (67%) | 12 (60%) | 14 (61%) |
| Esophagus | 0 | 1 (5%) | 1 (4%) |
| Bladder | 0 | 1 (5%) | 1 (4%) |
| Biliary tract | 0 | 1 (5%) | 1 (4%) |
| Unknown primary | 0 | 3 (15%) | 3 (13%) |
| Prior surgery | 3 (100%) | 15 (75%) | 18 (78%) |
| Prior radiotherapy | 2 (67%) | 16 (80%) | 18 (78%) |
| Prior chemotherapy | 3 (100%) | 20 (100%) | 23 (100%) |
Categorical data are shown as number of patients with percentage in parenthesis
Paclitaxel pharmacokinetics after i.v. administration
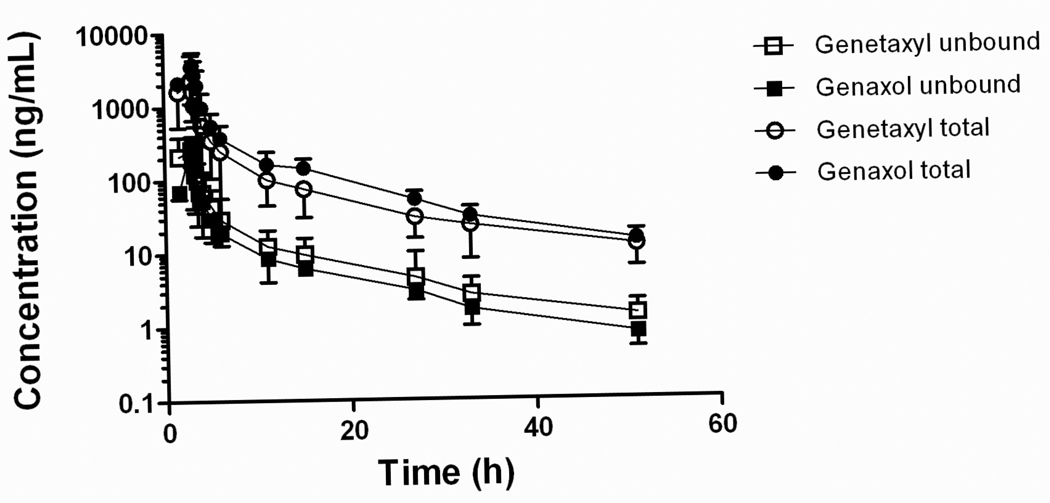
The median AUC and Cmax of total paclitaxel following the administration of i.v. Genaxol were 14.3 µg×h/mL and 4.43 µg/mL, respectively (Table 2), which values are comparable to the values found after i.v. Taxol [27]. The median AUC and Cmax of total paclitaxel following i.v. Genetaxyl were lower than those for i.v. Genaxol (Table 2), as a result of significantly increased clearance (P = 0.017). The median exposure to unbound paclitaxel was almost 2-fold higher after administration of i.v. Genetaxyl compared with i.v. Genaxol (1.17 versus 0.624 µg×h/mL, respectively) (Table 2), but this difference did not reach statistical significance (P = 0.43), presumably because of limited statistical power to detect differences using the small sample size studied. As expected, the AUC ratio for unbound to total paclitaxel for i.v. Genetaxyl was about 2 times higher than that for i.v. Genaxol (P = 0.0077; Table 2).
Table 2.
Pharmacokinetic parameters of paclitaxel after i.v. administrationa
| Parameter | Genaxol (n=3) | Genetaxyl (n=15) | P-valueb |
|---|---|---|---|
| Dose (mg/m2) | 175 | 175 | |
| Absolute dose (mg) | 242 (210–270) | 291 (263–340) | |
| CrEL in formulation (%) | 50 | 20 | |
| Cmax (µg/mL) | 4.43 (2.36–5.79) | 2.25 (0.960–5.80) | 0.10 |
| AUCt (µg×h/mL) | 14.3 (9.48–17.4) | 7.85 (3.71–21.2) | 0.13 |
| %CV | 29.0 | 51.5 | |
| AUCu (µg×h/mL) | 0.624 (0.462–0.930) | 1.17 (0.351–2.92) | 0.43 |
| %CV | 35.4 | 57.7 | |
| Fu (%) | 5.66 (1.99–9.14) | 10.6 (4.29–20.4) | <0.001 |
| AUCu/AUCt (%) | 4.87 (2.59–6.49) | 11.8 (8.14–16.5) | 0.0077 |
| CLu (L/h) | 387 (290–454) | 245 (113–836) | 0.43 |
| CLt (L/h) | 18.8 (13.9–22.1) | 36.8 (15.5–72.8) | 0.017 |
| T1/2 (h) | 11.1 (8.51–11.6) | 13.8 (9.51–16.5) | 0.059 |
Abbreviations: n, number of patients; CrEL, Cremophor EL; Cmax, peak plasma concentration; AUC, area under the plasma concentration versus time curve extrapolated to infinity; u, unbound paclitaxel; t, total paclitaxel; %CV, percent coefficient of variation in AUC; Fu, fraction unbound paclitaxel measured in all individual plasma samples; CL, systemic clearance; T1/2, half-life of the terminal phase.
All data are shown as median value for total paclitaxel, unless indicated otherwise, with range in parenthesis.
Nonparametric Mann-Whitney U test.
Pharmacokinetics and bioavailability of oral paclitaxel
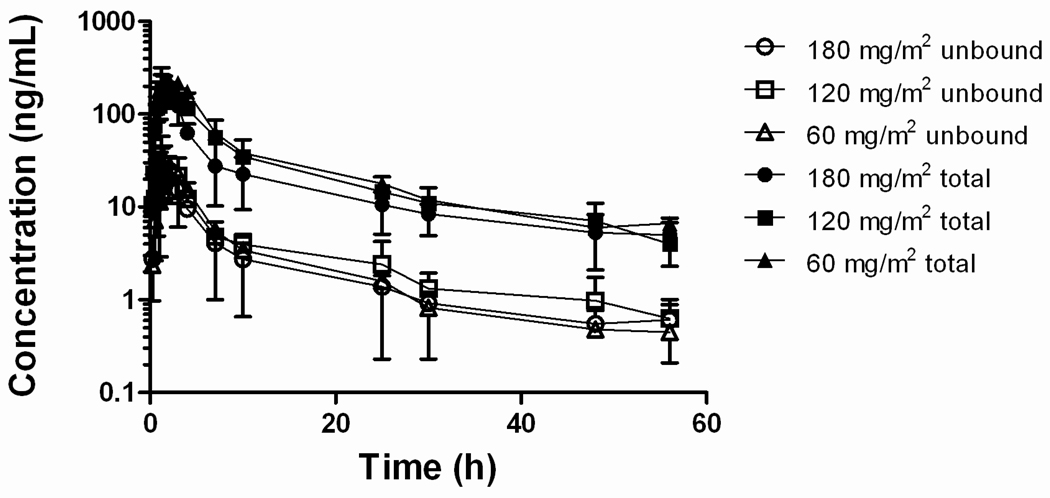
After oral administration of Genetaxyl at doses of 60, 120, and 180 mg/m2, the median total paclitaxel AUC values were 1.29, 1.60, and 1.85 µg×h/mL (Table 3), respectively, suggesting a less than proportional increase in systemic exposure to total paclitaxel with increasing doses. The corresponding values for the apparent oral bioavailability, however, were not statistically significantly dependent on the oral dose when calculated either based on data for total paclitaxel (overall median, 30.1%; P = 0.73) or unbound paclitaxel (overall median, 30.6%; P = 0.087).
Table 3.
Pharmacokinetic parameters of paclitaxel after oral administration of Genetaxyl given in combination with oral cyclosporin A (10 mg/kg)a
| Parameter | 60 mg/m2 (n=6) | 120 mg/m2 (n=6) | 180 mg/m2 (n=6) |
|---|---|---|---|
| Absolute dose (mg) | 108 (98–117) | 198 (180–222) | 296 (270–320) |
| Tmax (h) | 1.50 (1.05–2.00) | 2.50 (1.25–3.00) | 2.01 (1.00–4.00) |
| Cmax (µg/mL) | 0.185 (0.147–0.350) | 0.206 (0.129–0.424) | 0.290 (0.222–0.529) |
| AUCt (µg×h/mL) | 1.29 (1.12–1.64) | 1.60 (1.03–2.41) | 1.85 (0.865–9.51) |
| %CV | 14.5 | 37.8 | 63.9 |
| AUCu/AUCt (%) | 14.2 (9.95–21.9) | 12.5 (10.0–20.1) | 8.61 (7.42–10.1) |
| Bioavailability (%) | |||
| Unbound | 39.5 (16.1–67.5) | 27.6 (17.6–51.7) | 25.9 (16.7–76.9) |
| Total | 42.2 (22.3–56.0) | 29.8 (14.9–42.3) | 30.1 (29.8–65.5) |
| T1/2 (h) | 19.7 (14.5–23.2) | 17.9 (14.3–21.6) | 20.8 (14.4–24.9) |
Abbreviations: n, number of patients; Cmax, peak plasma concentration; AUC, area under the plasma concentration versus time curve extrapolated to infinity; u, unbound paclitaxel; t, total paclitaxel; %CV, percent coefficient of variation in AUCt; CL, systemic clearance; T1/2, half-life of the terminal phase.
All data are shown as median value for total paclitaxel, unless indicated otherwise, with range in parenthesis.
Adverse events
Toxicities observed following one course of oral paclitaxel with CsA were generally mild (grade 1–2), except that one patient developed grade 3 neutropenia and another patient developed grade 3 leukopenia after treatment with oral Genetaxyl at a dose of 180 mg/m2 (Table 4). The low degree of non-hematological toxicity after oral Genetaxyl is possibly the result of the extensive premedication regimen that was used. Six patients experienced grade 3–4 myelosuppression after i.v. Genetaxyl, whereas no such side effects were observed for the patients receiving i.v. Genaxol. Although the study was not designed to detect statistically differences in the incidence and severity of hematological toxicities, it is possible that the increased exposure to unbound paclitaxel observed in patients receiving i.v. Genetaxyl compared with i.v. Genaxol is related to this differential toxicity. One patient experienced a septic shock after i.v. Genaxol, but this was not considered a drug-related adverse event.
Table 4.
Major toxicities observed for oral and i.v. Genetaxyl and i.v. Genaxol
| Side effect | Genetaxyl | Genaxol | |||
|---|---|---|---|---|---|
| Cycle 1 (oral; 60, 120 or 180 mg/m2) n=20 | Cycle 2 (i.v.; 180 mg/m2) n=18 | (i.v.; 180 mg/m2) n=3 | |||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–4 | |
| Leukopenia | 5 (25) | 1 (5)* | 8 (44) | 2 (11) | 0 |
| Neutropenia | 4 (20) | 1 (5)* | 6 (33) | 4 (22) | 0 |
| Anemia | 6 (30) | 0 | 10 (56) | 0 | 1 (33)** |
| Diarrhea | 2 (10) | 0 | 0 | 0 | 0 |
| Nausea | 2 (20) | 0 | 2 (11) | 0 | 1 (33)** |
Patients from 180 mg/m2 cohort had grade-3 adverse events. These 2 patients were not the same.
Adverse event was graded as 2 in each category.
Discussion
The current study reports on the oral bioavailability and pharmacokinetics of total and unbound paclitaxel after oral administration of a novel paclitaxel formulation containing only 20% CrEL given in the presence of CsA. A prior study showed that a large fraction of both paclitaxel and CrEL was recovered unchanged in fecal samples after oral administration of Taxol [21], suggesting that the co-solvent CrEL could limit absorption of orally administrated paclitaxel by forming micelles entrapping the drug, without being itself absorbed [28]. Based on this finding, it was expected that the systemic exposure to paclitaxel after oral administration of Genetaxyl, containing 60% less CrEL as compared with Taxol, would be higher than those after administration of Taxol. Unfortunately, a group of patients receiving oral Taxol in the current study was not included to allow for a direct comparison, owing to limited patient resources.
Although it is difficult to compare pharmacokinetic data across different trials, a number of interesting points were noted. Firstly, the median time to peak concentration of paclitaxel following oral Genetaxyl at 60 mg/m2 was 1.5 hours, consistent with prior results showing that this parameter is lower when paclitaxel is administered in a formulation with lower concentrations of CrEL (1.5–2.9 versus 3.3–4.1 hours) [11,21]. This suggest that paclitaxel is absorbed more rapidly when the formulation contains no or less CrEL; it is possible that the rate of absorption of paclitaxel is affected by concentration-dependent entrapment of paclitaxel in CrEL micelles, thereby preventing paclitaxel from crossing the gastrointestinal membrane.
Secondly, values for peak concentration and AUC at the currently studied dose levels for oral Genetaxyl were similar to those reported previously in a study using oral Taxol at similar dose levels [17]. These results indicate that CrEL may play a more important role in affecting the initial rate of gastrointestinal absorption than processes taking place subsequently. Indeed, it is possible that the micellar effects of CrEL limit the absorption of paclitaxel in a time-dependent fashion, and that these effects are diminished with time due to enzymatic and/or chemical hydrolysis of the excipients within the gastrointestinal tract, as suggested previously [11].
Thirdly, the estimated oral bioavailability of paclitaxel following the oral administration of Genetaxyl at a dose of 60 mg/m2 was slightly higher than the relative oral bioavailability reported for generic Taxol given at the same oral dose (42% vs 31%) [17], and a similar difference between the two formulations was noted for an oral dose of 180 mg/m2 (30 vs 21%) [17]. It should be pointed out that the reduction in CrEL content of the currently studied Genetaxyl formulation resulted in much smaller values for paclitaxel AUC after i.v. administration compared to those obtained for generic Taxol, and resulted in a higher apparent bioavailability of paclitaxel after oral Genetaxyl after correction for differences in dose. The faster clearance of total paclitaxel following administration of i.v. Genetaxyl compared with i.v. Genaxol is consistent with data reported recently for a comparison of i.v. ABI-007 (nanoparticle-albumin bound paclitaxel containing no CrEL) and i.v. Taxol [29]. The underlying mechanism for the altered clearances between the two formulations is not entirely clear but is presumably associated with differential distribution to tissues of relevance to paclitaxel elimination such as the liver [30] and, subsequently, more rapid metabolism of paclitaxel when low CrEL levels are present in the circulation.
As mentioned previously, CrEL causes an apparent nonlinear disposition profile of total paclitaxel after i.v. Taxol, resulting in disproportional increases in plasma concentrations with increasing dose, and this process hampers the ability to calculate absolute oral bioavailability when i.v. Taxol is used as a reference. In the current study, it was noted that the bioavailability estimates for the Genetaxyl formulation were similar between calculations performed on the basis of unbound drug or total drug. This notion is consistent with the finding that the fraction unbound paclitaxel and the AUC ratio for unbound to total paclitaxel is similar after oral and i.v. administration of Genetaxyl. Furthermore, the median fraction unbound paclitaxel, about 11%, was similar to simulated in vivo values that were reported previously for paclitaxel in the absence of CrEL [22].
As observed previously for oral Taxol, it was noted that systemic exposure to paclitaxel after oral Genetaxyl was not proportionately increasing with increases in dose. These results appear to indicate that there is a maximum in the availability of paclitaxel for transfer across the intestinal barrier. It is unclear whether this dose-dependence is caused by increasingly larger amounts of CrEL being administered with increasing doses, but this possibility is likely given the amount of CrEL administered concurrently with Genetaxyl at a dose of 180 mg/m2. Regardless of the underlying mechanism, the observed dose-dependence in systemic exposure can likely be ameliorated by applying low dose schedules in conjunction with short-interval dosing of the oral Genetaxyl-CsA combination. Further evaluation of such dosing schedules with oral administration of Genetaxyl with CsA is warranted.
Fig. 1.
Plasma concentration-time profile of total and unbound paclitaxel following a 3-hour i.v. infusion of Genaxol or Genetaxyl at dose of 175 mg/m2 . Data are shown as mean values (symbols) with standard deviation (error bars).
Fig. 2.
Plasma concentration-time profile of total and unbound paclitaxel after oral administration of Genetaxyl at doses of 60, 120, and 180 mg/m2. Data are shown as mean values (symbols) with standard deviation (error bars).
Acknowledgments
This project has been funded in part with federal funds from the National Cancer Institute, under contract # N01-CO-12400 (ER Gardner). This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, Bethesda, MD. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Estevez LG, Munoz M, Alvarez I, Fernandez Y, Garcia-Mata J, Ruiz-Borrego M, et al. Evidence-based use of taxanes in the adjuvant setting of breast cancer. A review of randomized phase III trials. Cancer Treat Rev. 2007;33:474–483. doi: 10.1016/j.ctrv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Chu Q, Vincent M, Logan D, Mackay JA, Evans WK. Taxanes as first-line treatment for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2005;50:355–374. doi: 10.1016/j.lungcan.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Supl 6):S3–S11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Hait WN, Rubin E, Goodin S. Tubulin-targeting agents. Cancer Chemother Biol Response Modif. 2003;21:41–67. doi: 10.1016/s0921-4410(03)21003-6. [DOI] [PubMed] [Google Scholar]
- 5.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi R, Kumai T, Matsumoto N, Watanabe M, Kamio K, Suzuki S, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97:83–90. doi: 10.1254/jphs.fp0040603. [DOI] [PubMed] [Google Scholar]
- 7.Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH. MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005;116:824–829. doi: 10.1002/ijc.21013. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari SB, Amiji MM. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol. 2006;6:3215–3221. doi: 10.1166/jnn.2006.440. [DOI] [PubMed] [Google Scholar]
- 9.Peltier S, Oger JM, Lagarce F, Couet W, Benoit JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006;23:1243–1250. doi: 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- 10.Dabholkar RD, Sawant RM, Mongayt DA, Devarajan PV, Torchilin VP. Polyethylene glycol-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int J Pharm. 2006;315:148–157. doi: 10.1016/j.ijpharm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Veltkamp SA, Thijssen B, Garrigue JS, Lambert G, Lalenmand, Binlich F, et al. A novel self-microemulsifying formulation of paclitaxel for oral administration to patients with advanced cancer. Br J Cancer. 2006;95:729–734. doi: 10.1038/sj.bjc.6603312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veltkamp SA, Alderden-Los C, Sharma A, Rosing H, Beijnen JH, Schellens JH. A pharmacokinetic and safety study of a novel polymeric paclitaxel formulation for oral application. Cancer Chemother Pharmacol. 2007;59:43–50. doi: 10.1007/s00280-006-0245-2. [DOI] [PubMed] [Google Scholar]
- 13.Desai PB, Duan JZ, Zhu YW, Kouzi S. Human liver microsomal metabolism of paclitaxel and drug interactions. Eur J Drug Metab Pharmacokinet. 1998;23:417–424. doi: 10.1007/BF03192303. [DOI] [PubMed] [Google Scholar]
- 14.Qadir M, O’Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 15.Hesselink DA, van Hest RM, Mathot RA, Bonthuis F, Weimar W, de Bruin RW, et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant. 2005;5:987–994. doi: 10.1046/j.1600-6143.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 16.Meerum Terwogt JM, Malingre MM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Koopman FJ, et al. Coadministration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res. 1999;5:3379–3384. [PubMed] [Google Scholar]
- 17.Malingre MM, Meerum Terwogt JM, Beijnen JH, Rosing H, Koopman FJ, van Tellingen O, et al. Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol. 2000;18:2468–2475. doi: 10.1200/JCO.2000.18.12.2468. [DOI] [PubMed] [Google Scholar]
- 18.Britten CD, Baker SD, Denis LJ, Johnson T, Drengler R, Siu LL, et al. Oral paclitaxel and concurrent cyclosporin A: targeting clinically relevant systemic exposure to paclitaxel. Clin Cancer Res. 2000;6:3459–3468. [PubMed] [Google Scholar]
- 19.Kruijtzer CM, Schellens JH, Mezger J, Scheulen ME, Keilholz U, Beijnen JH, et al. Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4508–4516. doi: 10.1200/JCO.2002.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Kruijtzer CM, Boot H, Beijnen JH, Lochs HL, Parnis FX, Planting AS, et al. Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann Oncol. 2003;14:197–204. doi: 10.1093/annonc/mdg078. [DOI] [PubMed] [Google Scholar]
- 21.Malingre MM, Schellens JH, Van Tellingen O, Ouwehand M, Bardelmeijer HA, Rosing H, et al. The co-solvent Cremophor EL limits absorption of orally administered paclitaxel in cancer patients. Br J Cancer. 2001;85:1472–1477. doi: 10.1054/bjoc.2001.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henningsson A, Karlsson MO, Vigano L, Gianni L, Verweij J, Sparreboom A. Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol. 2001;19:4065–4073. doi: 10.1200/JCO.2001.19.20.4065. [DOI] [PubMed] [Google Scholar]
- 23.Chao TC, Chu Z, Tseng LM, Chiou TH, Hsieh RK, Wang WS, et al. Paclitaxel in a novel formulation containing less Cremophor EL as first-line therapy for advanced breast cancer: a phase II trial. Invest New Drugs. 2005;23:171–177. doi: 10.1007/s10637-005-5863-8. [DOI] [PubMed] [Google Scholar]
- 24.Malingre MM, Beijnen JH, Rosing H, Koopman FJ, van Tellingen O, Duchin K, et al. The effect of different doses of cyclosporin A on the systemic exposure of orally administered paclitaxel. Anticancer Drugs. 2001;12:351–358. doi: 10.1097/00001813-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gardner ER, Liau CT, Chu ZE, Figg WD, Sparreboom A. Determination of paclitaxel in human plasma following the administration of Genaxol or Genetaxyl by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2170–2174. doi: 10.1002/rcm.2577. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer E, Verweij J, De Bruijn P, Loos WJ, Pillay M, Buijs D, et al. Measurement of fraction unbound paclitaxel in human plasma. Drug Metab Dispos. 2000;28:1141–1145. [PubMed] [Google Scholar]
- 27.Scripture CD, Szebeni J, Loos WJ, Figg WD, Sparreboom A. Comparative in vitro properties and clinical pharmacokinetics of Paclitaxel following the administration of taxol(r) and paxene(r) Cancer Biol Ther. 2005;4:555–560. doi: 10.4161/cbt.4.5.1664. [DOI] [PubMed] [Google Scholar]
- 28.Bardelmeijer HA, Ouwehand M, Malingre MM, Schellens JH, Beijnen JH, van Tellingen O. Entrapment by Cremophor EL decreases the absorption of paclitaxel from the gut. Cancer Chemother Pharmacol. 2002;49:119–125. doi: 10.1007/s00280-001-0394-2. [DOI] [PubMed] [Google Scholar]
- 29.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136–4143. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 30.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]