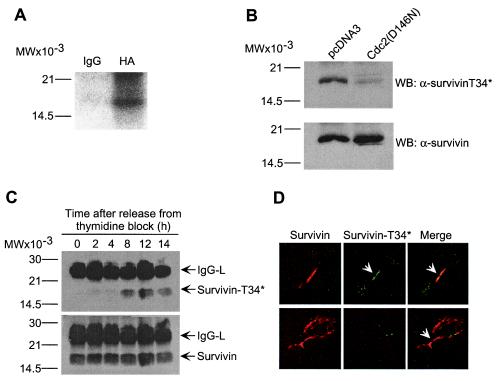
Figure 2.
Regulation of survivin phosphorylation by p34cdc2 in vivo. (A) In vivo phosphorylation of survivin. HeLa cells were transfected with HA-survivin, labeled with 200 μCi/ml 32PI and immunoprecipitated with control IgG or anti-HA followed by autoradiography. (B) Requirement of p34cdc2 kinase activity for survivin phosphorylation on Thr34. Endogenous survivin was immunoprecipitated from HeLa cells transfected with control pcDNA3 or kinase-dead p34cdc2 mutant (Cdc2(D146N)), and analyzed with α-survivinT34* or α-survivin, by Western blotting. No significant loss in cell viability was observed in HeLa cells expressing Cdc(D146N). (C) Kinetics of survivin phosphorylation on Thr34. Endogenous survivin was immunoprecipitated from cell cycle-synchronized HeLa cells at the indicated time intervals after thymidine release, and immunoblotted with α-survivinT34* or α-survivin. IgG-L, Ig light chain. (D) Localization of Thr34 phosphorylated survivin to midbodies. Endogenous survivin (Top) or HA-survivin(T34A) transfected in HeLa cells (Bottom) were labeled with mAb 8E2 to survivin (8) or an antibody to HA, respectively, plus α-survivinT34*. Binding of the primary antibodies was detected by addition of Texas Red (TR)-conjugated goat anti-mouse (survivin, red) and FITC-conjugated goat anti-rabbit (Thr34 phosphorylated survivin, green) antibodies. Coverslips were analyzed by confocal laser scanning microscopy. Image merging analysis is shown on the right. (A–C) Relative molecular weight markers are indicated on the left. WB, Western blotting.