Fig. 1.
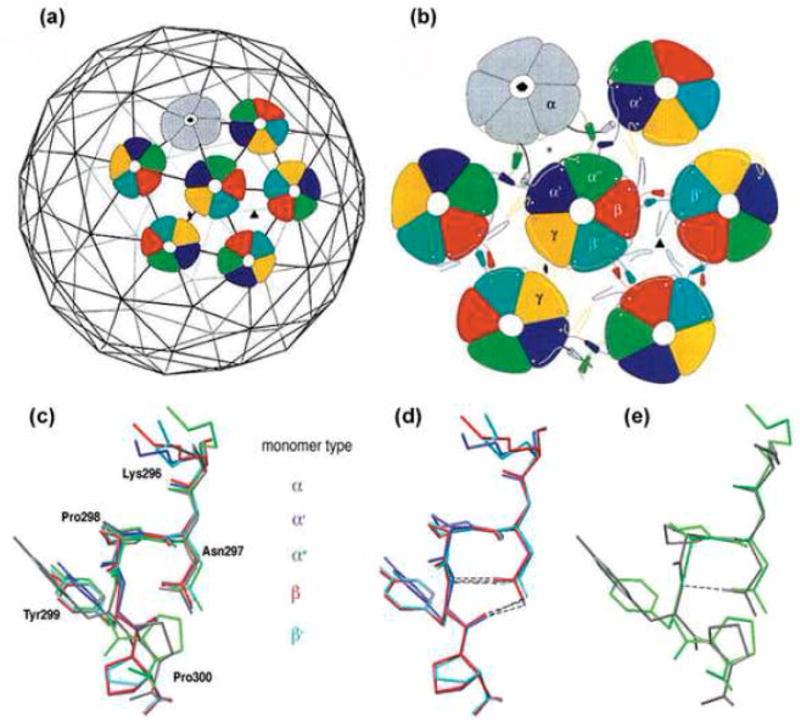
Top: Architecture of the virion shell (reprinted from2 with permission from Elsevier). (a) Arrangement of the pentavalent (grey) and hexavalent (coloured) pentamers on the T=7d icosahedral lattice. (b) Three distinct types of interactions between pentamers. α monomers (grey) of pentavalent pentamers and monomers, α′ and α″ of hexavalent pentamers (coloured) form a three-helix contact. The hexavalent pentamers interact through two-helix contact of monomers β- β′ and γ-γ. Bottom: The different conformations of the pentapeptide hinge. (c) Superimposition of the pentapeptide hinge of monomers α (grey), α′ (blue), α″ (green), β (red) and β′ (turquoise). Y299 and P300 have distinct orientations in the different monomers. The pentapeptide of γ monomers is not included, as its high B-factor indicates that it is less ordered.2 (d,e) The two well-ordered conformation groups. Superimposition of monomers β, α′ and β′ shows stabilization by two hydrogen bonds (d), while monomers α and α″ contain only one or no hydrogen bond, respectively (e). Superimpositions were prepared using Swiss-PDB-Viewer.23
