Fig. 5.
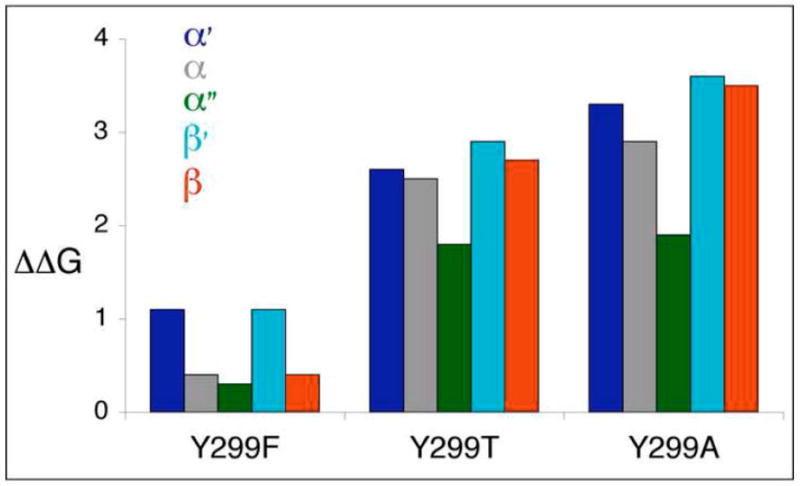
Calculated effect of mutations on binding. The predicted ΔΔG values of the mutations Y299F, Y299T, Y299A for each of the five tested chains, α (grey), α′ (blue), α″ (green), β (red) and β′ (turquoise) are shown (in Rosetta Units – an energy decrease of >1–1.5kcal/mol indicates significantly impaired binding). The effect of Y299F is negligible, while truncation of the sidechain to T, and even more to A, results in a significant loss of binding energy.
