ABSTRACT
OBJECTIVE
To determine primary care physician screening, treatment, and control rates for hypertension and to examine whether type of physician payment model affected these rates.
DESIGN
A cross-sectional chart abstraction study.
SETTING
Community health centres (salary), primary care networks (capitation), or traditional fee-for-service practices in Ontario.
PARTICIPANTS
A total of 135 primary care physicians, 45 from each of the 3 different models of care. Data were abstracted from 28 adult patient charts randomly selected from each physician.
MAIN OUTCOME MEASURES
Screening rates were based on the presence of at least 1 blood pressure reading in the past 3 years, treatment rates on the number of patients with hypertension treated with antihypertensive medication, and control rates on the number of patients with hypertension whose most recent blood pressure readings were below 140/90 mm Hg, below 130/80 mm Hg for patients with diabetes, or below 120/75 mm Hg for patients with renal disease.
RESULTS
Overall, 92.5% of all patients were screened for hypertension, 86.4% of patients with hypertension were treated with antihypertensive medications, and 44.9% of patients with hypertension had their blood pressure controlled. Mean screening rates were 90.6%, 93.5%, and 93.3% (P = .22), and after adjusting for sociodemographic factors and comorbid conditions, mean treatment rates were 90.9%, 81.0%, and 87.4% (P < .05) and mean control rates were 54.5%, 38.6%, and 41.6% (P < .05) for capitation, salary, and fee-for-service physicians, respectively.
CONCLUSION
Our results showed that although screening rates were similar between all 3 models, there were differences in treatment and control rates, with capitation physicians having the best treatment and control rates. Further investigation into whether this type of payment model results in improved chronic disease management for other chronic diseases and preventative care maneuvers will give support to health care policy makers who are moving toward capitation-type payment models for primary care delivery.
RÉSUMÉ
OBJECTIF
Examiner les taux de dépistage, de traitement et de contrôle de l’hypertension chez des médecins de première ligne et vérifier si le mode de rémunération du médecin affecte ces taux.
TYPE D’ÉTUDE
Étude transversale à partir de dossiers.
CONTEXTE
Centres de santé communautaires (salaire), réseaux de soins primaires (capitation) ou cliniques traditionnelles avec rémunération à l’acte de l’Ontario.
PARTICIPANTS
Un total de 135 médecins de première ligne, 45 de chacun des 3 modèles de soins. Les données proviennent de 28 dossiers choisis au hasard parmi les patients de chacun des médecins.
PRINCIPAUX PARAMÈTRES À L’ÉTUDE
Le taux de dépistage était basé sur la présence d’au moins une lecture de tension artérielle au cours des 3 dernières années, le taux de traitement sur le nombre d’hypertendus recevant une médication antihypertensive et le taux de contrôle sur le nombre d’hypertendus chez qui la dernière lecture de tension artérielle était inférieure à 140/90 mm Hg, inférieure à 130/80 mm Hg chez les diabétiques et inférieure à 120/75 mm Hg chez ceux souffrant de maladie rénale.
RÉSULTATS
Dans l’ensemble, 92,5 % des patients avaient eu un dépistage pour l’hypertension, 86,4 % des hypertendus recevaient des antihypertenseurs et 44,9 % des hypertendus avaient une tension artérielle contrôlée. Les taux moyens de dépistage étaient de 90,6, 93,5 et 93,3 % (P = 0,22) et, après ajustement pour les facteurs sociodémographiques et les affections associées, les taux moyens de traitement étaient de 90,9, 81,0 et 87,4 % (P < 0,05) et les taux moyens de contrôle de 54,5, 38,6 et 41,6 % (P < 0,05), respectivement pour les médecins des groupes capitation, salaire et rétribution à l’acte.
CONCLUSION
Nos résultats indiquent que même si les 3 modèles avaient des taux de dépistage semblables, il y avait des différences pour les taux de traitement et de contrôle, les médecins du groupe capitation ayant les meilleurs taux de traitement et de contrôle. Des recherches additionnelles pour déterminer si ce mode de paiement entraîne une amélioration dans le traitement d’autres maladies chroniques et dans les interventions de prévention pourraient appuyer la tendance de ceux qui élaborent les politiques des soins de santé et qui favorisent un modèle de paiement du type capitation pour la dispensation des soins primaires.
Canada has a universal-access medical system in which provincial governments act as insurers to pay for physician services, hospitalizations, investigations, and procedures. In the past few years there has been a national primary care reform movement to examine alternative models of primary care delivery. Traditionally, primary care physicians have been paid according to a fee-for-service (FFS) model, without government support for overhead costs or for allied health professionals. Current primary care reforms, however, are moving toward capitation-type payment models, and “mixed-model” approaches based on capitation payments, in which physicians are paid based on the number of patients enrolled with them rather than on a per-visit basis as is the case in FFS models.
Primary care networks (PCNs) were introduced in Ontario in 2001. Physicians practising in PCNs are paid by capitation, and PCNs have been the springboard for newer reform models that pay physicians by capitation-based payment schemes. Physicians practising in PCNs are also given funding for the transition to electronic medical records, continuing medical education reimbursements, and in some cases funding support to hire nurse practitioners. Newer capitation models have also included incentive payments for reaching certain ideal targets for preventative care. However, no incentive payments related to hypertension detection or management have been introduced.
Another model of primary care delivery in Canada is the community health centre (CHC). These have been in operation in Canada since the 1920s, and there are currently more than 50 CHCs in Ontario. Community health centres are non-profit, community-governed organizations, and primary care physicians in CHCs are paid salaries with benefits. They work in interdisciplinary teams, which include nurses, nurse practitioners, and many other types of allied health professionals. Community health centres have funding and capacity to provide health promotion and illness prevention services, and often have special programs for particular groups. These include members of linguistic or cultural groups; individuals who live in remote, underserviced communities; individuals with low incomes; individuals who are homeless; and the elderly.1
The incentives for all 3 models of care are different and might lead to variation in quality of care. For FFS physicians the incentive is to provide more patient visits, as physicians are paid per visit. For capitation physicians the incentives include having healthy patients who are less likely to require visits and providing less unnecessary care, as income is fixed regardless of how often each patient sees the physician.2,3 For salaried physicians there is no financial incentive to work more hours or see more patients; however, salaried physicians tend to work in underserviced or high-need populations where more time is required per patient to address needs.4
Hypertension is an important modifiable risk factor for cardiovascular disease5 and among the leading risk factors for mortality around the world.6,7 Previous studies have shown that awareness and control of hypertension in Canada are lower than in the United States.8 This is surprising in a setting of universal access to health care in Canada, compared with the large number of uninsured citizens in the United States. A more recent study, however, has shown great improvements in awareness and control of hypertension in Ontario, Canada’s most populous province.9 We looked at current practice patterns of physicians in Ontario with respect to hypertension and compared salaried, capitation, and FFS physicians’ rates of screening, treatment, and control of hypertension.
METHODS
We sent requests for participation to all 54 CHCs and all 12 PCNs. For the FFS physicians, we approached 500 family physicians from a randomly generated list provided by the College of Family Physicians of Canada. Physicians who had been at their current practice sites for at least 3 years and who were working at least part-time (3 days per week) were eligible to participate in our study. A modest honorarium and the opportunity to receive MainPro-C continuing medical education credits from the College of Family Physicians of Canada were offered to participating physicians.
A computerized data collection abstraction tool and a detailed abstraction manual were developed. Nine nurse abstractors participated in a 1-day training session and abstracted data on test charts. Abstractions took place in physician offices directly onto secured laptop computers between November 2004 and September 2005. Ten percent of all the practices underwent validation with double abstraction. Agreement rates were very high at 92.8% total agreement (κ 0.84) for the presence of hypertension. Nurse abstractors randomly selected charts from each physician’s practice until data had been collected from 28 eligible patients. Patients were eligible for inclusion if they were 38 years of age or older as of the date of abstraction; they were regular patients of the participating physician (defined by at least 2 of the following: seen by that physician the most, had a complete physical performed by that physician, or registered under that physician); they were still in the practice and had a valid Ontario health card number; they had first visited the participating physician at least 3 years before the date of chart abstraction; and they had been seen at least twice during the 3 years preceding the date of chart abstraction.
Data were collected from progress notes, laboratory results, and consultation notes from the 3 years before the date of abstraction and, when available, from the cumulative patient profile. Patients were classified as having hypertension if a physician-assigned diagnosis was recorded in their charts, they had prescriptions for antihypertensive medications in the context of elevated blood pressure readings, or if their recorded blood pressures met the criteria for diagnosis laid out in the Canadian Hypertension Education Program guidelines (systolic blood ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg).10 The number of visits, all blood pressure readings recorded in the charts, all cardiovascular-related prescriptions in the 3 years before the date of abstraction, as well as the presence or absence of cardiovascular-related conditions, dates of birth, sex, and health card numbers were collected.
Screening rates were calculated based on the number of patients with at least 1 blood pressure measurement documented in their charts. Treatment rates were calculated based on the number of patients with hypertension prescribed at least 1 antihypertensive medication. The most recent blood pressure measurement was used to assess control of hypertension; patients with hypertension whose most recent blood pressure readings were below 140/90 mm Hg, below 130/80 mm Hg for patients with diabetes, or below 120/75 mm Hg for patients with chronic renal disease were considered to have controlled hypertension.
Using the physician as the unit of analysis, a sample size calculation was done to detect differences in proportions of patients screened and treated using a 1-way ANOVA (analysis of variance) test. We assumed mean screening rates of 60% by FFS physicians and 70% by PCN and CHC physicians, and mean treatment rates of 60% by FFS physicians and 70% by PCN and CHC physicians. Based on previous reports, we expected a prevalence of hypertension of 22%11 (5 to 6 patients with hypertension per physician) and a control rate for patients with hypertension of 16%.11 Furthermore we assumed a within-arm standard deviation of 15% (thus estimating that the 95% confidence interval surrounding most screening and treatment rates in the FFS arm could vary from 30% to 90%). We used 45 physicians in each of 3 models to give us a power of at least 90% to detect a significant difference in mean screening and treatment rates between the groups using a significance level of .05. To maximize the accuracy of the proportion of patients screened and treated appropriately, we included 25 patients per physician, inflated by 10% to 28 patients per physician to account for patients who might have invalid health cards.
After the data were collected and analyzed, the actual power was calculated using 1-way ANOVA tests at a significance level of .05 based on actual means.
Quantitative analysis of the abstracted data was performed comparing the 3 different models of care and looking at physician characteristics, patient characteristics, and hypertension screening, treatment, and control rates using χ2, F statistic of ANOVA, Wilcoxon rank sum tests, and Fisher exact tests where relevant. Logistic regression analyses adjusting for age, sex, socioeconomic status (SES), urban versus rural residence, presence of diabetes, and presence of 0, 1, 2, 3, or more cardiovascular-relevant comorbid conditions were performed for important outcome comparisons. All data analyses were conducted using SAS, version 9.13. Postal code information was obtained by linking encrypted health card numbers to the Registered Persons Database. Using postal codes, rural versus urban status was determined using Statistics Canada’s definition of rural and small town (population less than 10 000) versus noncensus metropolitan areas (population at least 10 000 but less than 100 000) and census agglomerations (population at least 100 000).12 Income quintiles based on 2001 census data derived from postal code census geography provided by Statistics Canada were used to estimate SES.13,14
This study received ethics approval from the institutional review board at Sunnybrook Health Sciences Centre in Toronto, Ont.
RESULTS
Physician demographics
Of the 54 CHCs and 12 PCNs in operation at the time of the study, physicians from 21 different CHCs and 7 PCNs agreed to participate. Among the physicians participating in the study, there were no significant differences between the 3 models of care in physician sex, average age, urban versus rural practice, academic versus community practice, and average number of physicians per practice. Nurses and nurse practitioners were not as available to FFS physicians, and PCN physicians had more completely computerized patient records. Physicians in FFS practice reported the highest average weekly work hours (46.1); CHC physicians worked the fewest hours (39.3) (Table 1).
Table 1.
Physician demographics by model of care: A) Number and proportion of physicians with various characteristics; B) Mean age, age at graduation, weekly hours worked, and number of physicians in group.
| A) | |||||
|---|---|---|---|---|---|
| MODEL
|
|||||
| PHYSICIAN CHARACTERISTICS | CHC, N (%)(N = 45) | FFS, N (%)(N = 45) | PCN, N (%)(N = 45) | OVERALL, N (%)(N = 135) | P VALUE |
| Female | 23 (51.1) | 15 (33.3) | 22 (48.9) | 60 (44.4) | .18 |
| Hospital privileges* | 32 (71.1) | 35 (77.8) | 45 (100.0) | 112 (83.0) | < .01 |
| Rural practice | 18 (40.0) | 10 (22.2) | 18 (40.0) | 46 (34.1) | .12 |
| Community practice (vs academic practice) | 40 (88.9) | 44 (97.8) | 40 (88.9) | 124 (91.9) | .23 |
| Solo practice* | ≤ 5 | 17 (37.8) | 16 (35.6) | 34 (25.2) | < .01 |
| Nurse available in practice* | 39 (86.7) | 20 (44.4) | 30 (66.7) | 89 (65.9) | < .01 |
| Nurse practitioner available in practice* | 45 (100.0) | ≤ 5 | 25 (55.6) | 73 (54.1) | < .01 |
| Computerized progress notes* | ≤ 5 | ≤ 5 (11.1) | 38 (84.4) | 46 (34.1) | < .01 |
| Computerized cumulative patient profiles* | 27 (60.0) | 10 (22.2) | 37 (82.2) | 74 (54.8) | < .01 |
| B) | |||||
| MODEL
|
|||||
| PHYSICIAN CHARACTERISTICS | CHC, MEAN (SD) (N = 45) | FFS, MEAN (SD) (N = 45) | PCN, MEAN (SD) (N = 45) | OVERALL, MEAN (SD) (N = 135) | P VALUE |
| Physician age in 2005 | 46.3 (8.6) | 46.8 (7.9) | 50.2 (8.6) | 47.7 (8.5) | .06 |
| Physician age at graduation | 27.0 (3.6) | 26.3 (1.9) | 26.0 (2.3) | 26.4 (2.7) | .21 |
| Physician weekly hours worked* | 39.3 (10.2) | 46.1 (13.9) | 43.0 (11.5) | 42.8 (12.2)† | .04 |
| Physicians in group | 4.0 (1.6) | 4.0 (7.3) | 4.0 (3.1) | 4 (4.6) | .89 |
CHC–community health centre, FFS–fee-for-service, PCN–primary care network.
P < .05, statistically significant difference between models.
This information was not available for 13 of the physicians.
Patient demographics and characteristics
In all, 3773 adult patients were included in our study (1249, 1263, and 1261 patients in CHC, FFS, and PCN models, respectively). The average patient age was 55 years and 58% were female; there were no significant differences in age or sex between models. Socioeconomic status did differ between models. There were more even income distributions among FFS and PCN patients; more CHC patients were in the lower income quintiles (Table 2). Presence of cardiovascular-related comorbidities was similar between practice settings for all of the patients and for the patients with hypertension. Smoking status was available for just under 80% of the patients. Half of the patients overall were non-smokers, but CHC patients had higher smoking rates compared with FFS and PCN patients (Table 2).
Table 2.
Patient demographics by model of care: A) All patients; B) Patients with hypertension.
| A) | |||||
|---|---|---|---|---|---|
| MODEL
|
|||||
| PATIENT CHARACTERISTICS | CHC, MEAN (SD) | FFS, MEAN (SD) | PCN, MEAN (SD) | OVERALL, MEAN (SD) | P VALUE |
| Female, % | 58.7 (16.0) | 54.6 (16.5) | 59.6 (18.7) | 57.6 (17.1) | .33 |
| Age at date of abstraction, y | 54.1 (5.7) | 56.3 (4.2) | 55.5 (3.9) | 55.3 (4.7) | .08 |
| Socioeconomic status, % | |||||
| • Income quintile 1 (lowest)* | 40.0 (23.3) | 15.4 (13.1) | 16.9 (12.8) | 24.1 (20.4) | < .01 |
| • Income quintile 2 | 21.8 (12.7) | 22.1 (9.7) | 19.3 (10.9) | 21.1 (11.2) | .44 |
| • Income quintile 3* | 14.7 (8.8) | 20.0 (10.6) | 18.9 (11.1) | 17.9 (10.4) | .04 |
| • Income quintile 4* | 12.6 (11.8) | 19.5 (9.7) | 20.3 (11.4) | 17.5 (11.5) | < .01 |
| • Income quintile 5 (highest)* | 10.8 (11.4) | 23.0 (13.6) | 24.5 (20.4) | 19.4 (16.7) | < .01 |
| Cardiovascular-related comorbidities, % | |||||
| • Dyslipidemia | 31.5 (13.3) | 34.4 (16.5) | 33.3 (12.7) | 33.1 (14.2) | .63 |
| • Stroke | 2.4 (2.8) | 2.7 (3.3) | 2.3 (2.8) | 2.5 (3.0) | .80 |
| • Transient ischemic attack | 2.0 (3.1) | 1.5 (2.1) | 1.7 (2.2) | 1.7 (2.5) | .64 |
| • Angina | 5.1 (6.0) | 6.0 (5.2) | 6.2 (6.3) | 5.8 (5.8) | .67 |
| • Myocardial infarction | 4.9 (4.9) | 4.4 (5.6) | 4.8 (6.3) | 4.7 (5.6) | .88 |
| • Congestive heart failure | 2.3 (3.3) | 1.7 (2.9) | 1.4 (2.4) | 1.8 (2.9) | .35 |
| • Atrial fibrillation | 2.1 (3.3) | 2.8 (4.3) | 2.6 (3.0) | 2.5 (3.6) | .63 |
| • Coronary artery bypass graft | 1.4 (2.7) | 1.7 (2.5) | 2.1 (4.0) | 1.7 (3.1) | .65 |
| • Previous angioplasty or stent | 1.8 (2.6) | 1.3 (2.8) | 2.5 (3.4) | 1.9 (3.0) | .15 |
| • Peripheral vascular disease | 3.3 (5.0) | 3.2 (3.7) | 2.0 (3.2) | 2.8 (4.0) | .23 |
| • Diabetes | 12.9 (8.9) | 13.5 (8.2) | 11.5 (8.9) | 12.6 (8.6) | .54 |
| • Renal disease | 1.2 (2.0) | 1.7 (3.0) | 2.6 (3.7) | 1.9 (3.0) | .08 |
| • Hypothyroidism | 7.0 (6.0) | 6.0 (5.7) | 9.3 (7.1) | 7.4 (6.4) | .05 |
| • Current smoker* | 31.6 (15.6) | 23.9 (15.3) | 25.9 (13.9) | 27.1 (15.2) | .04 |
| B) | |||||
| MODEL
|
|||||
| PATIENT CHARACTERISTICS | CHC, MEAN (SD) | FFS, MEAN (SD) | PCN, MEAN (SD) | OVERALL, MEAN (SD) | P VALUE |
| Female, % | 60.6 (22.0) | 49.8 (21.8) | 57.0 (23.5) | 55.8 (22.7) | .07 |
| Age at date of abstraction, y | 61.0 (6.8) | 62.1 (6.4) | 63.7 (4.3) | 62.2 (6.0) | .11 |
| Socioeconomic status, % | |||||
| • Income quintile 1 (lowest)* | 42.2 (28.1) | 17.3 (19.8) | 18.6 (17.3) | 26.0 (24.9) | < .01 |
| • Income quintile 2 | 25.0 (18.6) | 24.1 (16.1) | 20.0 (17.3) | 23.1 (17.4) | .36 |
| • Income quintile 3* | 11.6 (11.9) | 20.0 (17.4) | 18.8 (16.9) | 16.8 (15.9) | .02 |
| • Income quintile 4* | 11.8 (15.7) | 20.3 (19.8) | 20.9 (19.3) | 17.7 (18.7) | .04 |
| • Income quintile 5 (highest)* | 9.4 (12.2) | 18.3 (15.8) | 21.8 (24.8) | 16.5 (19.0) | < .01 |
| Cardiovascular-related comorbidities, % | |||||
| • Dyslipidemia | 55.0 (19.0) | 53.5 (22.9) | 58.4 (18.8) | 55.6 (20.3) | .51 |
| • Stroke | 4.0 (6.9) | 5.5 (7.3) | 7.1 (8.9) | 5.5 (7.8) | .17 |
| • Transient ischemic attack | 3.4 (5.5) | 3.4 (5.4) | 3.9 (5.8) | 3.5 (5.5) | .88 |
| • Angina | 10.6 (12.8) | 12.4 (11.6) | 12.8 (11.7) | 11.9 (12.0) | .64 |
| • Myocardial infarction | 9.9 (11.0) | 9.8 (12.2) | 11.1 (14.6) | 10.3 (12.6) | .87 |
| • Congestive heart failure | 4.9 (7.4) | 4.1 (6.9) | 3.6 (6.9) | 4.2 (7.1) | .70 |
| • Atrial fibrillation | 3.1 (6.0) | 4.0 (5.9) | 5.4 (9.5) | 4.1 (7.3) | .21 |
| • Coronary artery bypass graft | 3.3 (5.4) | 2.8 (5.9) | 5.1 (8.0) | 3.8 (6.6) | .34 |
| • Previous angioplasty or stent | 3.3 (6.3) | 5.9 (8.4) | 5.8 (8.7) | 5.0 (7.9) | .21 |
| • Peripheral vascular disease | 7.0 (9.1) | 7.0 (9.9) | 4.5 (7.3) | 6.2 (8.9) | .29 |
| • Diabetes | 28.0 (16.9) | 28.2 (16.5) | 30.0 (20.7) | 28.7 (18.0) | .84 |
| • Renal disease* | 2.0 (4.5) | 4.6 (9.2) | 6.8 (10.0) | 4.5 (8.5) | .03 |
| • Hypothyroidism | 10.3 (11.3) | 7.0 (8.6) | 11.6 (12.1) | 9.6 (10.9) | .12 |
| • Current smoker | 30.3 (22.5) | 20.8 (18.3) | 21.6 (22.1) | 24.2 (21.3) | .07 |
CHC–community health centre, FFS–fee-for-service, PCN–primary care network.
P < .05, statistically significant difference between models.
Screening rates
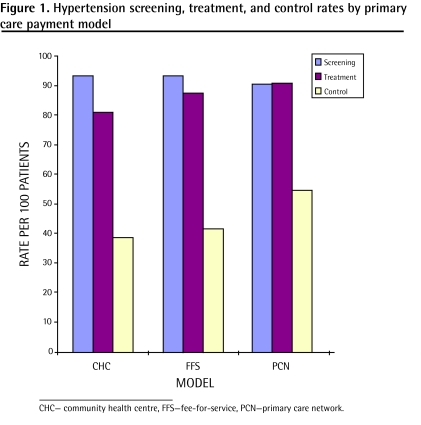
Screening rates for hypertension were high in all 3 models (Figure 1), with 93% of patients having at least 1 blood pressure measurement recorded, 78% having at least 2 blood pressure measurements recorded, and 61% having at least 3 blood pressure measurements recorded in the past 3 years. Although screening rates using 2 or 3 blood pressure measurements taken in the past 3 years were lower for PCN physicians, their patients had fewer total visits while maintaining a similar percentage of visits in which blood pressure measurements were taken, compared with the other 2 models (Table 3).
Figure 1.
Hypertension screening, treatment, and control rates by primary care payment model
Table 3.
Hypertension screening rates and number of visits in the past 3 years by model of care
| MODEL
|
|||||
|---|---|---|---|---|---|
| SCREENING | CHC, MEAN (SD) | FFS, MEAN (SD) | PCN, MEAN (SD) | OVERALL, MEAN (SD) | P VALUE |
| At least 1 BP visit in past 3 y, % | 93.5 (8.9) | 93.3 (7.2) | 90.6 (9.6) | 92.5 (8.7) | .22 |
| Average no. of visits in the past 3 y,* % | 14.9 (3.7) | 12.9 (4.2) | 9.9 (2.2) | 12.6 (4.0) | < .01 |
| Proportion of total visits in the past 3 y in which BP was measured | 43.3 (13.1) | 48.7 (15.0) | 47.0 (17.0) | 46.3 (15.2) | .22 |
BP–blood pressure, CHC–community health centre, FFS–fee-for-service, PCN–primary care network.
P <.05, statistically significant difference between models.
Treatment rates
The prevalence of hypertension was 34% overall, and among the patients with hypertension, 86% of patients were treated with antihypertensive medications, with PCN patients having the highest treatment rates (91%) and CHC patients having the lowest treatment rates (81%) (Figure 1). Patients with hypertension in PCNs were also more commonly on at least 2 antihypertensive medications (60%), whereas CHC patients had the lowest percentage of hypertension patients on 2 or more antihypertensives (48%). Although fewer CHC patients took acetylsalicylic acid and lipid-lowering medications, the difference compared with the other models was not statistically significant. The distribution of types of antihypertensive medications prescribed was relatively similar between models for most of the subclasses of antihypertensives, although fewer CHC patients were taking β-blockers and angiotensin II receptor blockers (Table 4).
Table 4.
Adjusted treatment and control rates for patients with hypertension by model of care
| MODEL
|
|||||
|---|---|---|---|---|---|
| TREATMENTS AND MEASURES OF CONTROL | CHC, MEAN (SD) | FFS, MEAN (SD) | PCN, MEAN (SD) | OVERALL, MEAN (SD) | ANOVA P VALUE |
| Patients with hypertension, %* | 33.7 (10.5) | 36.7 (8.8) | 30.3 (8.4) | 33.6 (9.3) | .01 |
| Currently taking at least 1 BP medication, %* | 81.0 (4.9) | 87.4 (4.5) | 90.9 (4.9) | 86.4 (4.6) | .01 |
| Currently taking at least 2 BP medications, %* | 47.7 (6.2) | 60.0 (5.3) | 59.8 (5.9) | 55.8 (6.6) | < .01 |
| Currently taking ASA, % | 34.9 (10.5) | 38.7 (9.9) | 37.9 (7.4) | 37.2 (9.4) | .67 |
| Currently taking lipid-lowering medication, % | 36.4 (8.1) | 44.5 (6.3) | 46.4 (6.9) | 42.4 (7.6) | .06 |
| Types of medication, % | |||||
| • ACE inhibitors | 47.8 (4.2) | 56.5 (5.0) | 57.1 (4.0) | 53.8 (5.0) | .06 |
| • Angiotensin II receptor blockers* | 8.9 (4.6) | 19.1 (4.5) | 15.1 (3.9) | 14.3 (4.2) | .03 |
| • β-Blockers* | 25.2 (3.0) | 32.2 (5.7) | 34.6 (4.2) | 30.7 (4.6) | .03 |
| • Calcium channel blockers | 24.1 (7.8) | 28.2 (6.2) | 29.9 (7.0) | 27.4 (7.1) | .19 |
| • Diuretics | 50.7 (6.0) | 50.3 (4.5) | 53.9 (4.8) | 51.6 (5.2) | .67 |
| Last individual BP reading below target,*† % | 38.6 (5.9) | 41.6 (6.8) | 54.5 (5.6) | 44.9 (6.7) | < .01 |
| Average no. of visits in the past 3 y* | 19.1 (1.2) | 16.9 (1.3) | 14.0 (1.0) | 16.7 (1.4) | < .01 |
| Proportion of total visits in the past 3 y in which BP was measured | 63.4 (3.8) | 67.8 (3.5) | 66.5 (3.7) | 65.9 (3.8) | .22 |
ACE–angiotensin-converting enzyme, ANOVA–analysis of variance, ASA–acetylsalicylic acid, BP–blood pressure, CHC–community health centre, FFS–fee-for-service, PCN–primary care network.
P < .05, statistically significant difference between models.
Target BP < 140/80 mm Hg, < 130/80 mm Hg for patients with diabetes, and < 120/75 for patients with renal disease.
Control rates
The mean control rate for all of the patients with hypertension was 45%, and PCN physicians had the highest control rate (55%) (Table 4 and Figure 1).
Adjustment and power
Controlling for age, sex, SES, urban or rural residence, and presence of diabetes or other cardiovascular-related comorbidities did not affect the comparative percentages or statistical significance of the comparisons of treatment and control rates between models. We had a power of 0.33, 0.78, and 0.93 for detecting differences between screening, treatment, and control rates, respectively.
DISCUSSION
As anticipated, patients of PCN physicians were seen the least often, but the percentage of visits where blood pressure measurements were taken and the screening rates were similar for all 3 groups. Although the incentive for capitation physicians is to have healthier patients in their practices, we did not find significant differences in the mean age or presence of cardiovascular-related comorbidities between the 3 groups, and SES was similar between FFS and PCN patients.
Patients in CHCs were more commonly in the lowest income quintile and had the highest smoking rates, which might have contributed to the lower rates of blood pressure control seen in patients of CHC physicians. However, patients of CHC physicians had the lowest rates of being prescribed both 1 or 2 antihypertensives, and although there very well could be adherence issues, with patients of lower SES being unable to afford medications, our measurement was based on what was prescribed and not what was actually taken. Whether SES affects physician prescribing rates could not be measured here. Interestingly, while FFS and PCN physicians had similar treatment rates, PCN physicians had higher control rates. This suggests that PCN physicians might be more effectively managing hypertension despite seeing their patients less often.
Despite the differences we found between the different models of care, our study demonstrates that overall current practice patterns of physicians in Ontario with respect to hypertension have shown great improvement from what was previously reported.11 This is consistent with recently published studies using administrative databases showing increasing prevalence15 of patients diagnosed with hypertension and decreasing mortality16 among patients diagnosed with hypertension in Ontario, suggesting improved detection and treatment of hypertension.
Although 85% of the general population report having had their blood pressure measured within the past 2 years,17 screening rates for hypertension by primary care physicians have not previously been reported. We found that more than 90% of the randomly selected adult patients in our study had had at least 1 blood pressure measurement taken in the past 3 years, suggesting that primary care physicians are adequately screening for high blood pressure among the patients in their practice.
Eighty-six percent of the patients in our study with hypertension were treated with at least 1 antihypertensive medication. This is considerably higher than the 58% treatment rate found in the Canadian Heart Health Survey,11 conducted over a decade and an half ago, and slightly higher than the 80% treatment rate found in the 2006 Ontario Survey on the Prevalence and Control of Hypertension.9 This higher treatment rate can be expected, as our treatment rates were measured in patients who visited their physicians’ offices and included patients aged 38 and older. Other studies, in contrast, measured treatment rates at the community level, which would include patients found to have hypertension who do not necessarily have physicians or who have not been to see their physicians. In addition, the other studies included patients aged 18 and 20 years and older, respectively, and treatment rates in both studies increased in the older age categories.
Our control rates were also higher, with 45% of patients with hypertension having their blood pressure readings below target, compared with the Canadian Heart Health Survey finding of 16% treated and controlled. We do know that the use of polytherapy for treating hypertension has been steadily increasing in the past decade,18 and thus it is likely that our substantially higher control rate reflects a true improvement. However, our control rate is not nearly as high as the 66% treated and controlled found in the Ontario Survey.9 The Ontario Survey conducted in the community used a BpTRU automated blood pressure measurement machine, and measurements were taken by community nurses outside of the physician office setting; thus they might not be subject to the “white coat hypertension” bias that physician office readings might be subject to. This discrepancy certainly warrants further investigation and highlights the difficulties of comparing hypertension prevalence treatment and control studies conducted in different settings with different blood pressure measurement techniques.
Limitations
Our study relied on willingness of physicians to participate, thus overall measures of screening, treatment, and control rates for hypertension might be better than they are for the general primary care physician population, as better-performing physicians might be more willing to participate. The same bias, however, would apply to physicians in all 3 models of care, and thus comparison between models should not be substantially affected. Second, our study was limited to patients who see physicians. However, less than 6% of Ontarians report not having family physicians, and it has been estimated that less than 25% do not visit primary care physicians at least once a year.19 Thus, within a 2- or 3-year time period, it is likely that most Ontarians have at least 1 visit to their physicians. Third, whether our improved control rates are a result of improved recognition by physicians of the importance of hypertension, resulting in more aggressive treatment, or the fact that patients are more motivated or compliant with treatment cannot be answered by our study, but the factors that have led to this improvement warrant further investigation. Fourth, while we used a prescription for an antihypertensive as a proxy measure for treatment, we were unable to assess differences in lifestyle counseling treatment of hypertension between physicians. In addition, while we used the most recent blood pressure reading to assess control of hypertension, we were unable to adjust for factors such as time since diagnosis or time on and adherence to blood pressure medication. Last, PCNs were one of the first capitation models introduced in Ontario, and, as such, physicians who agreed to participate in this new model of care might be more forward-thinking or higher-performing physicians than the average primary care physician, thereby contributing to their higher performance here.
Conclusion
Our study was an initial look at comparing salaried, capitation, and FFS primary care payment models. For patients with hypertension, the PCN capitation model of care performed best. The results of this study should help to inform policy makers on the evaluation of alternative payment models. Whether a capitation-type payment system for primary care delivery results in similarly positive results for other chronic disease conditions and quality-performance measures warrants further investigation.
Acknowledgment
This study was funded by a grant from the Primary Health Care Transition Fund of the Ontario Ministry of Health and Long-term Care (MOHLTC) (Grant No. G03-02807). This work was completed independent of the funders, and they had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the MOHLTC. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the Institute for Clinical Evaluative Sciences or the MOHLTC is intended or should be inferred.
EDITOR’S KEY POINTS
In the past few years there has been a national primary care reform movement to examine alternative models of primary care delivery. This study compared rates of hypertension screening, treatment, and control for physicians in traditional fee-for-service practices with those in 2 other models of care–community health centres and the newer primary care networks.
Screening rates were similar between models, but mean treatment and control rates showed significant differences (P < .05); primary care networks had the highest rates, while community health centres had the lowest. Controlling for age, sex, socioeconomic status, urban or rural residence, and presence of cardiovascular-related comorbidities did not affect the comparative percentages or statistical significance of the comparisons.
Despite the differences found between the different models of care, this study demonstrates that overall current practice patterns of physicians in Ontario with respect to hypertension have shown great improvement from what has been previously reported.
POINTS DE REPÈRE DU RÉDACTEUR
Au cours des toutes dernières années, il y a eu un effort national de réforme des soins primaires visant à évaluer des modèles alternatifs pour la dispensation des soins primaires. Cette étude a comparé les taux de dépistage, de traitement et de contrôle de l’hypertension observés chez les médecins rémunérés à l’acte, par rapport aux taux dans 2 autres modèles de soins, soient les centres de santé communautaires et les nouveaux réseaux de soins primaires.
Les taux de dépistage étaient semblables dans les 3 modèles, mais les taux moyens de traitement et de contrôle montraient des différences significatives (P < 0,05); les réseaux de soins primaires avaient les taux les plus élevés tandis que les centres de santé communautaires avaient les plus bas. Le fait de contrôler pour l’âge, le sexe, le statut socio-économique, l’habitat urbain ou rural et la présence de comorbidité de type cardiovasculaire ne modifiait pas les pourcentages comparatifs ni la valeur statistique des comparaisons.
Malgré ces différences entre les différents modèles de soins, cette étude montre que dans l’ensemble, les modèles de pratique actuels des médecins ontariens concernant l’hypertension se sont grandement améliorés par rapport à ce qui avait été observé Cet article a fait l’objet d’une révision par des pairs. antérieurement.
Footnotes
This article has been peer reviewed.
Contributors
Dr Tu, Ms Cauch-Dudek, and Dr Chen contributed to concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Association of Ontario Health Centres [website] Who we are. Toronto, ON: Association of Ontario Health Centres; 2005. [Accessed 2009 Jun 4]. Available from: www.aohc.org/aohc/index.aspx?CategoryID=1&lang=en-CA. [Google Scholar]
- 2.Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev. 2000;(3):CD002215. doi: 10.1002/14651858.CD002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosden T, Pedersen L, Torgerson D. How should we pay doctors? A systematic review of salary payments and their effect on doctor behaviour. Q J Med. 1999;92(1):47–55. doi: 10.1093/qjmed/92.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Devlin RA, Sarma S, Hogg W. Remunerating primary care physicians: emerging directions and policy options for Canada. Healthc Q. 2006;9(3):34–42. doi: 10.12927/hcq..18225. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275(20):1571–6. [PubMed] [Google Scholar]
- 6.Ezzati M, Lopex AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. The world health report 2002. Geneva, Switz: World Health Organization; 2002. [Google Scholar]
- 8.Joffres MR, Hamet P, MacLean DR, L’italien GJ, Fodor G. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens. 2001;14(11 Pt 1):1099–105. doi: 10.1016/s0895-7061(01)02211-7. [DOI] [PubMed] [Google Scholar]
- 9.Leenen FH, Dumais J, McInnis NH, Turton P, Stratychuk L, Nemeth K, et al. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ. 2008;178(11):1441–9. doi: 10.1503/cmaj.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, McAllister FA, Myers MG, McKay DW, Bolli P, Abbott C, et al. The 2005 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1—blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2005;21(8):645–56. [PubMed] [Google Scholar]
- 11.Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10(10 Pt 1):1097–102. doi: 10.1016/s0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 12.Du Plessis V, Beshiri R, Bollman R, Clemenson H. Definitions of “rural”. Agriculture and Rural Working Paper Series 2002;Working Paper No. 61; pp. 1–43. [Google Scholar]
- 13.Wilkins R. PCCF+ version 4D user’s guide: automated geographic coding based on the Statistics Canada postal code conversion files, including postal codes to December 2003. Ottawa, ON: Health Analysis and Measurement Group, Statistics Canada; 2003. [Google Scholar]
- 14.Mustard CA, Derksen S, Berthelot JM, Wolfson M. Assessing ecologic proxies for household income: a comparison of household and neighbourhood level income measures in the study of population health status. Health Place. 1999;5(2):157–71. doi: 10.1016/s1353-8292(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 15.Tu K, Chen Z, Lipscombe LL Canadian Hypertension Education Program Outcomes Research Taskforce. Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ. 2008;178(11):1429–35. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu K, Chen Z, Lipscombe LL Canadian Hypertension Education Program Outcomes Research Taskforce. Mortality among patients with hypertension from 1995 to 2005: a population-based study. CMAJ. 2008;178(11):1436–40. doi: 10.1503/cmaj.080096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statistics Canada. Canadian community health survey. Ottawa, ON: Statistics Canada, Health Statistics Division; 2001. [Google Scholar]
- 18.Campbell NR, McAlister FA, Duong-Hua M, Tu K. Polytherapy with two or more antihypertensive drugs to lower blood pressure in elderly Ontarians. Room for improvement. Can J Cardiol. 2007;23(10):783–7. doi: 10.1016/s0828-282x(07)70827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan BTB, Schultz SE. Supply and utilization of general practitioner and family physician services in Ontario. ICES investigative report. Toronto, ON: Institute for Clinical Evaluative Sciences; 2005. [Google Scholar]