Abstract
Aims
The role of ultraviolet C light (UVC)-induced phosphorylation of the eukaryotic initiation factor 2 (eIF2) in the regulation of cyclooxygenase-2 (COX-2) expression at both transcriptional and translational levels is investigated.
Main methods
Western analysis was used to determine COX expressions. Immunoprecipitation after [35S]-Met/Cys metabolic labeling was used to determine the rate for COX-2 synthesis and turnover. Quantitative real-time PCR was used to determine COX-2 mRNA levels. Ingenuity Pathways Analysis 6 was used for mapping COX-2 activation network.
Key findings
UVC induces COX-2 expression in wild-type mouse embryo fibroblasts (MEFS/S) and that the inducibility is reduced in MEFA/A cells in which the phosphorylation site, Ser-51 in the eIF2α, is replaced with a nonphosphorylatable Ala (S51A). UVC-induced transcription of COX-2 is delayed in MEFA/A cells, which correlates with NF-κB activation as previously reported (Wu, S, Tan, M, Hu, Y, Wang, JL, Scheuner, D, Kaufman, RJ, Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. The Journal of Biological Chemistry, 279, 34898–34902, 2004). The translational efficiency of COX-2 is higher in MEFA/A cells than in MEFS/S cells at 4 h, but not at 24 h post-UVC. The translation efficiency is correlated to the ratio of activated COX-2 binding protein HuR/TIAR. In addition, the newly synthesized COX-2 protein is more stable in MEFA/A cells than in MEFS/S cells. The results demonstrated a complex and dynamic regulation of COX-2 expression.
Significance
UVC induces a prolonged expression of COX-2. While transcriptional regulation of COX-2 expression is intensively studied, the role of translational regulation of COX-2 synthesis upon UVC-irradiation is not yet clear. This study elucidated a novel eIF2α phosphorylation-centered network for the regulation of COX-2 expression after UVC-irradiation.
Keywords: Ultraviolet light (UVC), Cyclooxygenase (COX), Eukaryotic initiation factor 2 (eIF2), Nuclear factor-κB (NF-κB), Human ELAV-like protein (HuR), TIA-1-related protein (TIAR)
Introduction
Cyclooxygenases (COXs) catalyze the rate-limiting step in the production of prostaglandins (PG) from arachnoic acid. COX-1 is a constitutively active housekeeping enzyme expressed at low levels in most tissues and acts as a housekeeping regulator of gastric and renal homeostasis (Smith et al.1996). COX-2 is the inducible isoform activated, amongst others, by inflammatory cytokines, oncogenes, growth factors and UV-radiation (Prescott and Fitzpatrick 2000). COX-2 expression is intricately regulated through multiple signaling pathways. Several transcription factor binding sites (CREB, C/EBP, TCF4, NFIL6, AP2, SP1 and NF-κB) in the COX-2 promoter region have been identified (Appleby et al. 1994). The most well studied mechanism for transcriptional activation of UV-induced COX-2 expression is the p38MAPK, which activates theCREB/ATF1 pathway (Tang et al. 2001; Ulivi et al. 2008). The nuclear factor-κB (NF-κB) was also shown to transcriptionally regulate COX-2 expression (Hung et al. 2004; Korkolopoulou et al. 2008; Lee et al. 2004; Mutoh et al. 2007; Ulivi et al. 2008). However the role of NF-κB in the UV-induced COX-2 expression is still not clear.
Expression of COX-2 is also translationally regulated. COX-2mRNA is locally regulated by the binding of a host of RNA-binding proteins to the AU-rich element (ARE) in the 3′-untranslated region (3′-UTR) of the COX-2 mRNA (Cok and Morrison 2001; Kedersha and Anderson 2002; Ristimaki et al. 1994). Two translational regulators of COX-2, human ELAV-like protein (HuR) and T-cell-restricted intracellular antigen 1 (TIA-1)-related protein (TIAR), were shown to locally regulate the translation of COX-2 mRNA upon UVC-irradiation (Tong et al. 2007; Van Dross et al. 2007). HuR and TIAR, are shown to increase or decrease the translation efficiency of the bound mRNA, respectively (Cok et al. 2003, 2004). UV-irradiation also inhibits global protein synthesis by inducing the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α), which activates NF-κB translationally via translational inhibition of IκBα synthesis (Deng et al. 2002, 2004; Wu et al. 2002, 2004). However, the mechanism for regulation of COX-2 expression via global translation inhibition is not clear. In this report, we show a mechanism of translational regulation of UVC-induced COX-2 expression both at global levels and that of RNA binding proteins.
In this report, we provide evidence that phosphorylation of eIF2α also plays an important role in the regulation of COX-2 expression after UVC-irradiation. Our results demonstrate eIF2α phosphorylation is required for UVC-induced expression of COX-2 at transcription and translation levels. Without eIF2α phosphorylation, the UVC-induced transcription activation of COX-2 is delayed, which correlates with NF-κB activation, as previously reported synthesis (Deng et al. 2002, 2004;Wu et al. 2002, 2004); while the translation efficiency of COX-2 is increased at the early stage, but not late stage, of the irradiation. The increased translation of COX-2 is correlated to the ratio of activated HuR/TIAR. In addition, newly synthesized COX-2 protein is more stable when eIF2α phosphorylation is abolished. These findings elucidate a complex and dynamic role of translation initiation in the regulation of COX-2 expression.
Material and methods
Cell culture
Wild type mouse embryo fibroblasts (MEFS/S) and mutated ones (MEFA/A), in which Ser 51 on the alpha subunit of the eukaryotic initiation factor (eIF2α) is mutated to a non-phosphorylatable Ala, were grown in 10% FBS enriched DMEM media (Cellgro) containing MEM essential and non-essential amino acids (Invitrogen). The cells were incubated at 37 °C.
UVC irradiation
UVC was generated from a 15 W UVC source (UVP). The intensity of the UVC was standardized by using a UV light meter (UVP) set at 3W/m2. The medium was withdrawn during irradiation.
Protein extraction
The irradiated cells were harvested at the indicated time-points post-irradiation using Nonidet P-40 lysis buffer (2% NP-40, 80 mM NaCl, 100 mM Tris–HCl, and 0.1% SDS) for total protein extraction. Cytoplasmic and nuclear proteins were extracted using NucBuster Protein Extraction Kit from Novagen (EMD Biosciences). Both extraction reagents were supplemented with protease inhibitors of Cocktail Set 3 from Calbiochem (EMD Biosciences). Protein concentrations were measured with a Protein DC assay kit (Bio-Rad).
Western analysis
Equal amounts of protein samples were subjected to SDS-PAGE and electroblotted to nitrocellulose membranes. The membranes were probed with rabbit polyclonal antibodies anti-COX-2 (sc-1747R, Santa Cruz), anti-COX-1 (sc-7950, Santa Cruz), goat polyclonal anti-TIAR (sc-1749, Santa Cruz), anti-hnRNP C1/C2 (sc-10037, Santa Cruz), mouse monoclonal anti HuR (sc-5261; Santa Cruz) and anti-β-actin antibodies (Sigma). After extensively washing with Tris-buffered saline plus Tween 20 (TBS–T), the membranes were incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies. The proteins were detected by using a SuperSignal™ chemiluminescent kit (Pierce).
Assay for COX-2 synthesis and turnover
Cells were UVC-irradiated (30 J/m2). At the indicated time points after irradiation, the cells were incubated with methionine/cysteine-free minimal essential medium (Cellgro) for 15 min and then pulse-labeled with Redivue pro mix [35S]-Met/Cys (100 µCi/mL) (Amersham Biosciences) for 30 min in Met/Cys-free minimal essential medium (Cellgro). After washing with phosphate buffer saline (PBS), the cells were harvested or continuously incubated in fresh complete medium for 2 h before harvesting. The cell extracts were prepared in radioimmunoprecipitation (RIPA) buffer (Tris–HCl: 50 mM, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate,150mM NaCl, and 1mM EDTA). The protein concentration was measured with a Protein DC assay kit (Bio-Rad). COX-2 was then immunoprecipitated from equal amounts of proteins using anti-COX-2 antibody (sc-1747R, Santa Cruz) and protein A-agarose (Vector). The immunoprecipitates were subjected to SDS-PAGE. The gel was stained with 0.2% Coomasie Blue R-250, treated with En3Hance (PerkinElmer Life Sciences) and vacuum dried. The amounts of [35S]-labeled COX-2 were then analyzed by autoradiography.
Assay for COX-2 stability
Cells were UVC-irradiated (30 J/m2). At the indicated time points after irradiation, the cells were harvested or treated with cyclohex-amide (100 µg/mL) for 1 h before harvesting. Total amount of COX-2 was determined by western blot analysis.
Total protein synthesis assay
The UVC-irradiated MEFs were pulse-labeled with [35S]-Met/Cys as described above. The [35S]-incorporation was analyzed by SDS-PAGE by loading equal amounts of proteins. The gel was stained with Coomassie Blue R-250 for total protein detection and treated with En3Hance (PerkinElmer Life Sciences). The gel was then dried for autoradiography.
Quantitative real-time PCR
Total RNA was extracted from UVC-treated cells by RNeasy Mini Kit (Qiagen). Complementary DNA was prepared from 1 µg of RNase free DNase treated RNA, using iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR reactions were prepared with iQ SYBR Green Super Mix (Bio-Rad) and 0.2 µM concentration of the following primers:
COX-2: 5′: GCTGTACAAGCAGTGGCAAA; 3′: CCCCAAAGATAGCATCTGGA.
β-actin: 5′: TATGGAATCCTGTGGCATCC; 3′: GTACTTGCGCTCAGGAGGAG.
The reaction was performed on iCycler (Bio-Rad) starting with incubation at 95 °C for 2.5 min followed by 40 cycles of 60 s at 95 °C, 30 s at 60 °C and 30 s at 72°. Data were analyzed with the comparative delta Ct method. Relative amounts of COX-2 mRNA were normalized to the levels of β-actin mRNA in each sample.
Computational analysis of the COX-2 activation pathway
The COX-2 regulatory pathways were identified based on previously published research from within the Ingenuity Pathway Analysis library of Ingenuity Pathway Analysis 6 (Ingenuity® Systems).
Results
Inducibility of COX-2 upon UVC is eIF2α phosphorylation dependent
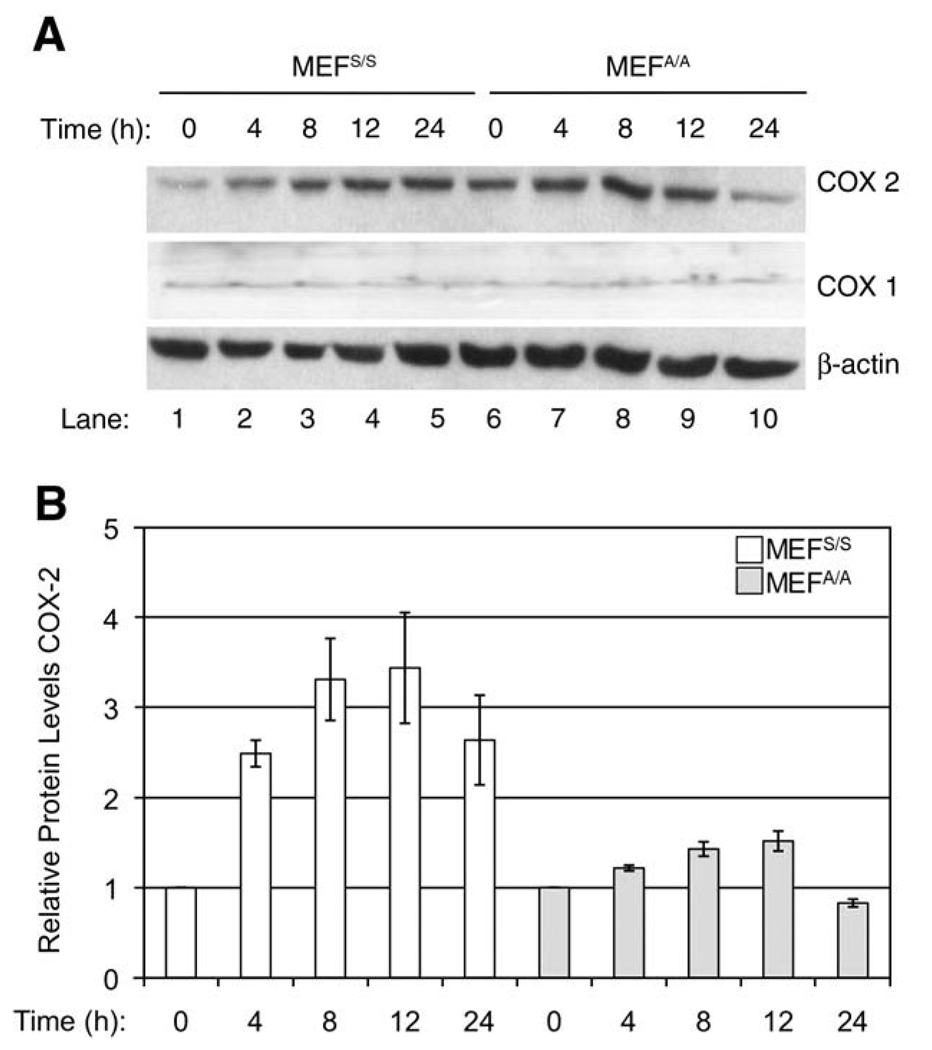
COX-2 expression is induced by NF-κB activation under various stimuli (Singer et al. 2003). Since NF-κB activation upon UVC-irradiation is regulated by translational inhibition of IκB synthesis, we have assessed the role of translation on the UVC-induced COX-2 expression. We first examined whether eIF2α phosphorylation plays a role in UVC-induced expression of COX-2. MEF cells with wild-type eIF2α (MEFS/S) or with a Ser-51→Ala mutation at the phosphorylation site in eIF2α (MEFA/A) were used in the experiments (Wu et al. 2002, 2004). The dose of 30 J/m2 was selected to keep consistency with previous experiments, where this dose was used to induce both eIF2α phosphorylation and NF-κB activation (Wu et al. 2002, 2004). Western blot analysis demonstrated that COX-2 expression was increased 1.5 to 2.1-fold from 4 to12h(Fig.1, Panel A, Lanes 2–4 vs.1; Panel B) in MEFS/S cells, whereas COX-2 increased only 0.4 to 0.8-fold in the same time period in MEFA/A cells after UVC-irradiation (Fig. 1, Panel A, Lanes 6–9; Panel B). In comparison to the 12 h time point the COX-2 levels were reduced in both cell lines at 24 h post-UVC (Fig. 1, Panel A, Lanes 5 vs.4 and 10 vs. 9; Panel B). To determine whether elimination of eIF2α phosphorylation specifically impacts COX-2 expression, we analyzed COX-1 expression in UVC-treated MEFS/S and MEFA/A cells. Our data showed that COX-1 expression levels are the same in both cell lines before and after UVC-irradiation (Fig. 1). These results suggest that eIF2α phosphorylation may play a dual-role in the regulation of COX-2 expression upon UVC-irradiation. While translational inhibition leads to the activation of NF-κB and transcriptional activation of COX-2 expression in MEFS/S cells after UVC-irradiation, maintaining a high level of active eIF2 increases translational efficiency of COX-2 in MEFA/A cells with or without UVC-irradiation.
Fig. 1.
Effect of translation on UVC-induced COX-2 expression. The MEFS/S and MEFA/A cells were irradiated with UVC (30 J/m2) and then were harvested at the indicated time points. (A) The COX-2 and COX-1 protein levels were detected by western blot analysis using anti-COX-2 and anti-COX-1 antibodies respectively. A western blot analysis of β-actin was used to monitor the loading of proteins. (B) The relative intensities of COX-2 protein bands were quantified by ImageJ (v.1.31, NIH). Results represent the means ± SEM for three independent experiments and levels are expressed relative to the COX-2 levels at 0 h post-UVC-irradiation for both cell lines.
The phosphorylation of eIF2α regulates both translation efficiency and stability of COX-2 upon UVC-irradiation
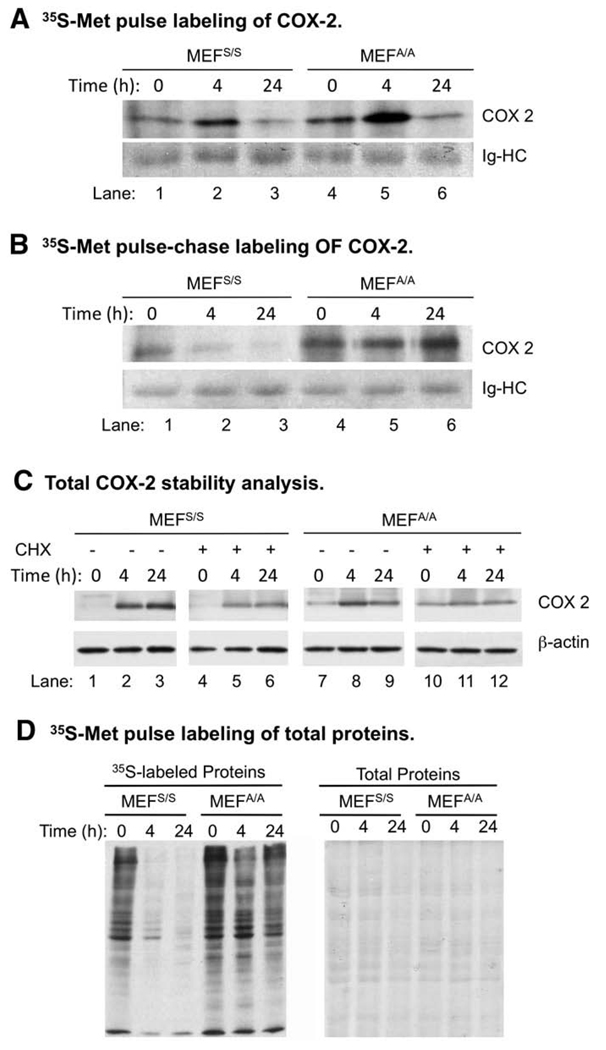
To elucidate the mechanism of translational regulation of COX-2 expression, we analyzed the kinetics of COX-2 synthesis and degradation using 35S-Met/Cys metabolic pulse labeling and pulse-chase methods. Our data show that the COX-2 synthesis and protein stability was higher in MEFA/A cells than in MEFS/S cells without UVC-irradiation (Fig. 2, Panels A and B, Lanes 4 vs. 1), which explains the higher background expression of COX-2 in MEFA/A cells (Fig. 1, Lanes 6 vs. 1). The COX-2 synthesis in both cell lines was increased at 4 h (Fig. 2, Panels A, Lanes 2, 5 vs. 1, 4) and then decrease at 24 h (Fig. 2, Panels A, Lanes 3, 6 vs. 1, 4) post-UVC-irradiation. The stabilities of newly synthesized COX-2 were similar (Fig. 2, B1/A1 vs. B4/A4; Table 1) and were decreased at 4 h in both cell lines after UVC-irradiation (Fig. 2, B2/A2 vs. B1/A1 and B5/A5 vs. B4/A4; Table 1). However, at 24 h post-UVC-irradiation, while the stability of newly synthesized COX-2was decreased in MEFS/S cells (Fig. 2, A3/B3 vs. A1/B1), it was slightly increased in MEFA/A cells (Fig. 2, B6/A6 vs. B4/A4; Table 1). These results suggest that eIF2α phosphorylation destabilized newly synthesized COX-2 in the late-stage of UVC-irradiation.
Fig. 2.
Translational efficiency and stability of COX-2 after UVC-irradiation. (A) MEFS/S and MEFA/A cells were irradiated with 30 J/m2 of UVC and then metabolically pulse-labeled with [35S]-Met/Cys for 20min at the indicated time points post irradiation. COX-2 protein was immunoprecipitated, subjected to SDS-PAGE. The gel was dried and the newly synthesized COX-2 was detected by autoradiograph. (B) Same cells were metabolically pulse-labeled with [35S]-Met/Cys and then cultured in complete medium without [35S]-Met/Cys for 2 h. COX-2was then immunoprecipitated from equal amounts of proteins and the [35S] labeled COX-2 was detected by autoradiography as described above. (C) MEFS/S and MEFA/A cells were treated with UVC (30 J/m2). At the indicated time points, the cells were treated or not treated with CHX (100αg/mL) for 1 h. Total COX-2 protein levels in the treated cells were determined by western blot analysis. (D) Equal amounts of [35S]-labeled proteins prepared in 2A were loaded on SDS-PAGE and dried. Newly synthesized proteins were detected by autoradiography (shown in left panel), and total amount of proteins were visualized by Coomassie Blue R-250 staining (right panel).
Table 1.
The translation efficiency and stability of COX-2 after UV-irradiation.
| Cell Lines | MEFS/S | MEFA/A | ||
|---|---|---|---|---|
| Time (h) | 0 4 | 24 | 0 4 | 24 |
| Translation efficiency (TE)* | 1 2.15 ± 0.01− | 0.53 ± 0.21$ | 1 2.31 ± 0.24− | 0.78 ± 0.28$ |
| Stability (Stab) * | 1 0.51 ± 0.11− | 0.32 ± 0.02− | 1 0.72 ± 0.09− | 0.85 ± 0.16$ |
| Stab/TE ** | 1 0.24 | 0.60 | 1 0.31 | 1.09 |
Numbers represent the averages and standard deviations of a duplicate experiment.
Numbers represent the ratios of the averages of the two experiments.
p<0.05
p<0.2, t-test.
To further determine the contribution of protein synthesis and degradation in the regulation of COX-2 level after UVC-irradiation, we analyzed the effect of cyclohexamide (CHX), a translation inhibitor, on UVC-induced COX-2 expression. One-hour pulse treatment of CHX was used to determine the effect of UVC on COX-2 protein stability in the two cell lines within a designed window of time. Surprisingly, our data show that CHX did not affect COX-2 levels in MEFS/S cells (Fig. 2, Panel C), while it significantly decreased them in MEFA/A cells after UVC-irradiation (Fig. 2, Panel C, Lanes 11, 12, vs. 8, 9). The maintenance of steady levels of COX-2 in MEFS/S cells was not due to the failure of CHX in inhibition of protein synthesis since IκBα levels were reduced in both cell lines in the same samples (data not shown). The results suggest that a combination of variation in protein synthesis and degradation pattern is accountable for the steadily increasing COX-2 levels detected in both cell lines after UVC-irradiation.
The new synthesis of COX-2was increased at 4 h post-UVC, which did not agree with the previously observed total protein synthesis in these cell lines (Wu et al. 2002, 2004). To confirm that COX-2 protein synthesis was increased while total protein synthesis was down regulated, we analyzed total protein synthesis in the same samples. Our data showed that translation is significantly reduced in MEFS/S cells, but not in MEFA/A cells after UVC-irradiation (Fig. 2, Panel D). These results agree with our previous report and demonstrate that COX-2 synthesis is not down regulated with the total protein synthesis after UVC-irradiation.
Translation inhibition activates COX-2 transcription
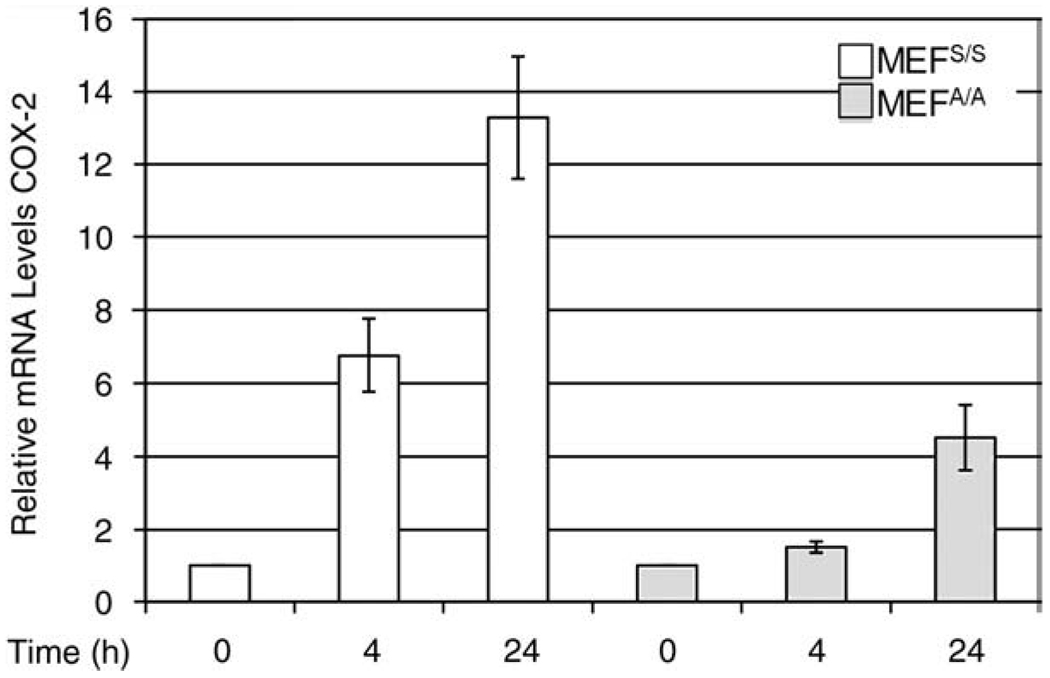
UV-irradiation inhibits protein synthesis through phosphorylation of eIF2α (Fig. 2, Panel D) (Wu et al. 2002). However our results indicated that newly synthesized COX-2 was increased in both cell lines at 4 h post-UVC (Fig. 2, Panel A), while total protein synthesis was down in the MEFS/S cells (Fig. 2, Panel D). To determine whether the increased expression of COX-2 is due to transcriptional activation of COX-2 expression, we assayed COX-2 mRNA levels with quantitative real time PCR in the total RNA of UVC irradiated MEFS/S and MEFA/A cells. The acquired data show that transcription levels of COX-2 increased more than 6-fold at 4 h and 13-fold at 24 h post-UVC in the MEFS/S cells, but showed only a modest increase at 4 h and a 5-fold increase in MEFA/A cells at 24 h (Fig. 3). The increase of transcription of COX-2 (Fig. 3) is correlated to the activation of NF-κB in the two cell lines after UVC irradiation (Laszlo and Wu 2008). The results suggest that the translational regulation of NF-κB activation plays a role in UVC-induced expression of COX-2.
Fig. 3.
UVC-induced eIF2α phosphorylation up-regulates COX-2 transcription. MEFS/S and MEFA/A cells were irradiated with UVC (30 J/m2). At the indicated time points, total mRNA was isolated and q-RT-PCR was used to determine the levels of COX-2 in the cells. Relative amounts of COX-2 transcripts from a triplicate experiment were normalized and expressed relative to the levels of β-actin housekeeping gene in each sample.
The phosphorylation of eIF2α affects the expression and activation of COX-2 mRNA-binding proteins
The UVC-induced translational inhibition was 100% and 50% countered in MEFA/A cells at 4 and 24 h post-UVC respectively (Wu et al. 2004). However, our data indicated that COX-2 protein synthesis was increased (Fig. 2, Panel A, Lanes 5 vs. 4) while the transcript levels were not significantly changed in the cells (Fig. 3) at 4 h post-UVC. In contrast, COX-2 protein synthesis was decreased significantly (Fig. 2, Panel A, Lanes 6 vs. 4), while transcript levels were increased 5-fold at 24 h (Fig. 3). These results suggest that the translation efficiency of COX-2 mRNA is regulated not only by eIF2α phosphorylation after UV-irradiation. To further elucidate the mechanism of translational regulation of COX-2 expression, we analyzed the extent of effect of UVC-induced eIF2α phosphorylation on the expression and localization of two COX-2 mRNA-binding proteins, HuR and TIAR. HuR is known to increase and TIAR to reduce translation efficiency of the bound mRNA. Both proteins are predominantly nuclear proteins but exert their roles of binding to the RNA in the cytoplasm (Cok et al. 2003; Jang et al. 2003; Kandasamy et al. 2005; Mazan-Mamczarz et al. 2003; Piecyk et al. 2000; Tong et al. 2007; Wang et al. 2000).
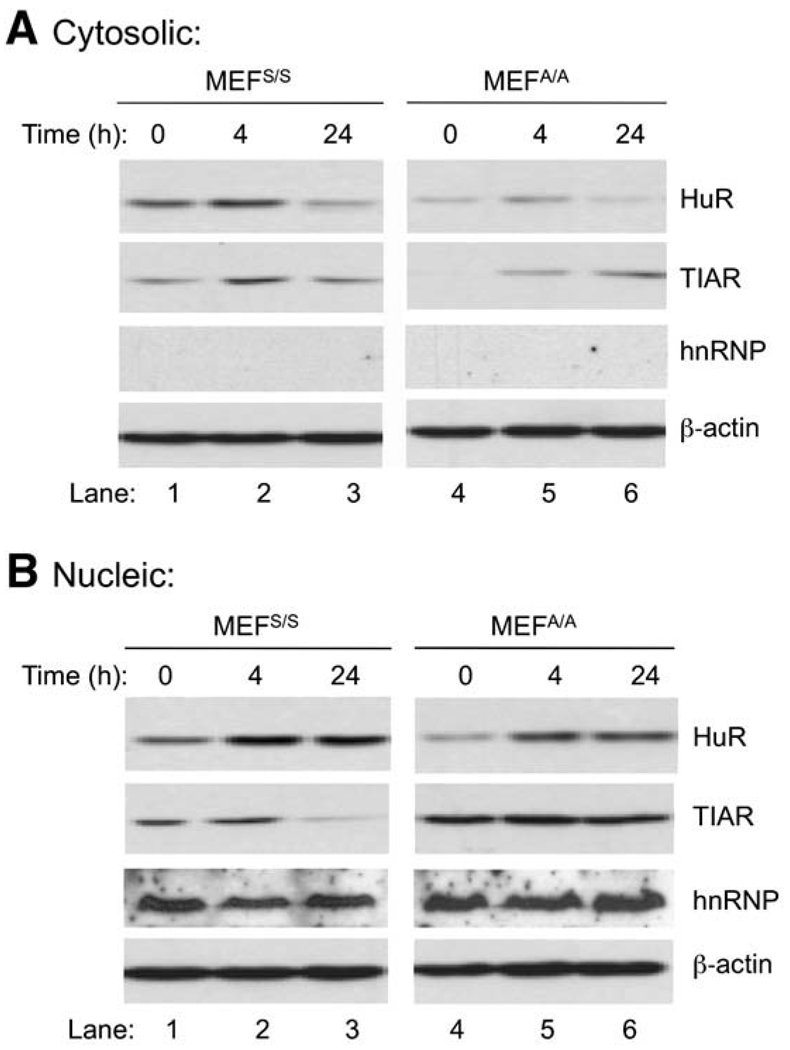
Western blot analysis shows that cytoplasmic levels of HuR/TIAR were increased in both MEFS/S and MEFA/A cells at 4 h post-UVC (Fig. 4, Panel A, Lanes 2, 5 vs. 1, 4). Since HuR and TIAR have the opposite effects in regulation of COX-2 translation, the result suggests that they likely do not play dominant roles in the regulation of COX-2 expression in the early stage of UVC-irradiation. Interestingly, at 24 h post-UVC, while cytoplasmic HuR was decreased in both cell lines (Fig. 4, Panel A, Lanes 3 and 6), TIAR was slightly increased above the base level in MEFS/S cells (Fig. 4, Panel A, Lanes 3 vs. 1), and was significantly increased in MEFA/A cells (Fig. 4, Panel A, Lanes 6 vs. 4). These results imply that translation of COX-2 could be less efficient in the late stage of UVC-irradiation due to a relatively lower HuR/TIAR ratio (Fig. 4, Panel A, Lanes 3, 6 vs. 1, 4). The results also suggest that the low efficiency of COX-2 translation (Fig. 2A) in the presence of higher levels of mRNA (Fig. 3) at 24 h post-UVC could be a combined effect of HuR/TIAR ratio and eIF2α phosphorylation.
Fig. 4.
Effect of UVC on post-transcriptional regulators of COX-2. MEFS/S and MEFA/A cells were irradiated with 30 J/m2 of UVC. At the indicated time points, the cytoplasmic (Panel A) and nuclear (Panel B) proteins were isolated. The proteins were subjected to western blot analysis using antibodies against HuR and TIAR. To monitor the loading and sub-cellular contamination, expression levels of β-actin and nuclear marker hnRNP C1/C2 were also determined using western blot analysis.
To further analyze the impact of eIF2α phosphorylation on regulation of HuR and TIAR expression and activation, we determined the amounts of the proteins in nucleus. Our data indicated that HuR levels were not reduced in either MEFS/S or MEFA/A cells after UVC-irradiation (Fig. 4, Panel B), which suggests that HuR expression was not correlated to eIF2α phosphorylation. In contrast to HuR, TIAR expression was not increased at 4 h post-UVC (Fig. 4, Panel B, Lanes 2, 5 vs. 1, 4), while the activity was increased (Fig. 4, Panel A, Lanes 2, 5 vs. 1, 4). Interestingly, TIAR expression was significantly decreased in MEFS/S cells but stayed the same in MEFA/A cells at 24 h post-UVC (Fig. 4, Panel B, Lanes 3, 6 vs. 2, 5), while activity is decreased in MEFS/S cells but increased in MEFA/A cells (Fig. 4, Panel A, Lanes 3, 6 vs. 2, 5). These results suggest that while HuR expression was not altered, TIAR expression was impacted by translation inhibition, especially in the late stage of UVC irradiation.
Discussion
Elucidating the role of translation regulation upon COX-2 activity may lead us to a better understanding of the mechanisms of current COX-2 targeting drugs and development of new therapeutics to treat diseases related to cancer, inflammation and pain. High expression levels of COX-2 in cancer cells indicate its significance in carcinogenesis (Chan et al.1999; Rundhaug and Fischer 2008). COX-inhibitors, such as non-steroidal anti-inflammatory drugs (NSAIDs), are known to inhibit tumor formation and metastasis (Marnett 1995; Smith et al. 2000). Celebrex, a COX-2 specific NSAID, was shown to significantly decrease the number of intestinal polyps in patients and was approved by the FDA for the treatment of familial adenomatous polypsis (FAP) (Takeda et al. 2003). The most common NSAIDs, such as aspirin, ibuprofen, meloxicam, acetaminophen and naproxen, act by inhibiting both COX-1 and COX-2. Although effective at reducing PG synthesis, these drugs have been shown to cause GI tract irritation due to the non-specific inhibition of COX-1 and its protective housekeeping functions, thus creating the interest in development of COX-2 specific inhibitors (Bingham et al. 2006). Some of these newer, promising candidates include valdecoxib, celecoxib, and rofecoxib (marketed under the brand names Bextra, Celebrex, and Vioxx respectively). However, a significant increase in heart attack and stroke resulted in the recall of Vioxx from the marketplace in 2004 and shed doubt on the viability of other similar COX-2 specific inhibitors (FitzGerald 2003). A new approach to COX-2 inhibition could lay in distinguishing the differences between COX-1 and COX-2 activation. COX-1 is an essential housekeeping enzyme, and prostaglandins whose synthesis involves COX-1 are responsible for maintenance and protection of the gastrointestinal tract. In contrast, prostaglandins whose synthesis involves COX-2 are responsible for severe symptoms such as inflammation and pain (Crofford 1997). Translation regulation, as shown by our study, could provide the needed distinction between COX-1 and COX-2 due to their differing activation patterns (Fig. 1, Panels A and B).
It was reported that LPS, TNFα, IL-1, IL-6, IFN and UV all could induce COX-2 expression through various, multiple and sometimes overlapping signaling pathways. LPS induces the TRAF6-NF-κB, the ERK-MSK-CREB (Eliopoulos et al. 2002) and the p38MAPK-ERK-C/EBP pathways (Chen et al. 2005). IL-1 and IFN-gamma signal through the cAMP-PKA-CREB cascade (Caivano and Cohen 2000; Maier et al. 1990; Wu et al. 2005), while TNFα-induced COX-2 expression was achieved by activating ERK and NF-κB (Dean et al. 2003). UV-irradiation induces COX-2 expression (Bachelor et al. 2002; Chen et al. 2001; Tang et al. 2001). It was previously suggested that UV induces COX-2 through the p38MAPK activated CREB/ATF1 transcriptionally regulated pathway (Tang et al. 2001; Tsatsanis et al. 2006). However, the COX-2 promoter region also contains the binding sites of various other transcription factors, including C/EBP, TCF4, NFIL6, AP2, SP1 and NF-κB (Appleby et al. 1994; Van Dross et al. 2007). We previously reported that UVC activates NF-κB by inducing phosphorylation of Ser 276 (Laszlo and Wu 2008), which is the targeting site of p38MAPK activated MSK-1 (Gustin et al. 2004; Han et al. 2003; Nagy et al. 2007). The role of NF-κB during UV-induced COX-2 expression is also evident because ER stress was suggested to be a COX-2 inducer (Hung et al. 2004) and UV is a well-known activator of ER stress (Rutkowski and Kaufman 2007; Wu and Kaufman 2006; Wu et al. 2002). There is evidence that p38 MAPK and NF-κB induce COX-2 through different signaling pathways (Tanabe and Tohnai 2002). While NF-κB induces activation only at the transcriptional level, p38MAPK activates COX-2 transcription through CREB/ATF1 and also takes part in the regulation of the COX-2 mRNA stability as well as translation efficiency (Singer et al. 2003). Message stability and translational efficiency are regulated through the employment of RNA-binding proteins that attach to the AU rich element (ARE) of the 3′UTR (Cok and Morrison 2001; Harper and Tyson-Capper 2008).
Previous studies demonstrate that the phosphorylation of eIF2α plays a role in the early stage of UVC-induced NF-κB activation (Deng et al. 2004;Wu et al. 2004).We now have systematically analyzed the extent of the effect of eIF2α phosphorylation on COX-2 expression upon UVC-irradiation. Our results demonstrate that eIF2α phosphorylation does not only regulate the global synthesis of COX-2 but also impacts the regulators and stabilities of COX-2 at both transcription and translation levels. The influence of eIF2α phosphorylation upon COX-2 expression was studied by using MEFS/S and MEFA/A cells. Our data show that the expression of COX-2 was induced much more in MEFS/S cells than in MEFA/A cells after UVC-irradiation (Fig. 1). The reduced inducibility of COX-2 in MEFA/A cells could be due to the higher level of background expression (Fig. 1 and Fig 2). While COX-2 protein synthesis rates were more than doubled at 4 h post-UVC in both cell lines (Fig. 2, Panel A and Table 1), the stabilities of the newly synthesized COX-2were reduced approximately 70% (Fig. 2, Panel B/A; Table 1, bottom row). The newly synthesized protein in MEFA/A cells appeared to have a lower impact on the increase of total amount of COX-2, probably due to the background COX-2 levels that were approximately 5 times higher in MEFA/A cells than in MEFS/S cells (Fig. 1, Panel A, Lane 6 vs. 1). Indeed, inhibition of protein synthesis by CHX affected more the total amount of COX-2 in MEFA/A cells than in MEFS/S cells (Fig. 2, Panel C), which correlated with the higher synthesis rate of newly synthesized COX-2 in MEFA/A cells (Fig. 2, Panel A). Interestingly, inhibition of protein synthesis did not appear to affect the total amount of COX-2 in MEFS/S cells and it only reduced them to background level in MEFA/A cells (Fig. 2, Panel C). In addition, after inhibiting new protein synthesis, the levels of COX-2 in both cell lines after UVC-irradiation were similar to the background level of COX-2 in MEFA/A cells. The results suggest that a steady amount of “matured” COX-2 may be more stable than the newly synthesized one. It will be interesting to further investigate whether COX-2 is stabilized after its association with the cell membrane.
While the protein synthetic rate is expected to be higher in MEFA/A cells at 0 and 4 h post-UVC, it was unexpected that the translation of COX-2was significantly inhibited in MEFA/A cells at 24 h post-UVC since our previous results demonstrated that translation was protected in these cells due to the non-phosphorylatable mutation of eIF2α (Wu et al. 2004). To further determine the mechanism of UVC-induced expression of COX-2, we analyzed mRNA levels of COX-2 using the real time quantitative PCR (RT-qPCR). We found that transcriptional activation of COX-2 is significantly reduced in MEFA/A cells compared to the wild type cells (Fig. 3). Although at this point we cannot quantitatively analyze the contribution of transcription and RNA stability towards the total COX-2 mRNA levels, these transcript levels can be associated with NF-κB activation patterns in the two cell-lines after UVC-irradiation. The high mRNA levels at 24 h post irradiation shown by the RT-PCR in the MEFA/A cells appears to be the result of the action of NF-κB which we showed to be active during this period despite the inability of UVC to block translation (Laszlo andWu 2008;Wu et al. 2004). Since the COX-2 transcript levels and translation rates upon UVC-irradiation were not correlated to each other, we analyzed the levels of two COX-2 mRNA-binding proteins, HuR and TIAR, in the two cell lines after UVC treatment. HuR stimulates COX-2 mRNA translation, while TIAR inhibits it (Tong et al. 2007). Our data demonstrate that HuR expression could bypass UVC-induced and eIF2α phosphorylation-mediated translation inhibition (Fig. 4), whereas TIAR expression was significantly inhibited at 24 h post-UVC (Fig. 4, Panel B, Lane 3). The differential expression of HuR and TIAR altered the ratio of activated HuR/TIAR (Fig. 4, Panel A) and impacted the translation efficiency of COX-2 after UVC-irradiation (Fig. 2, Panel A).
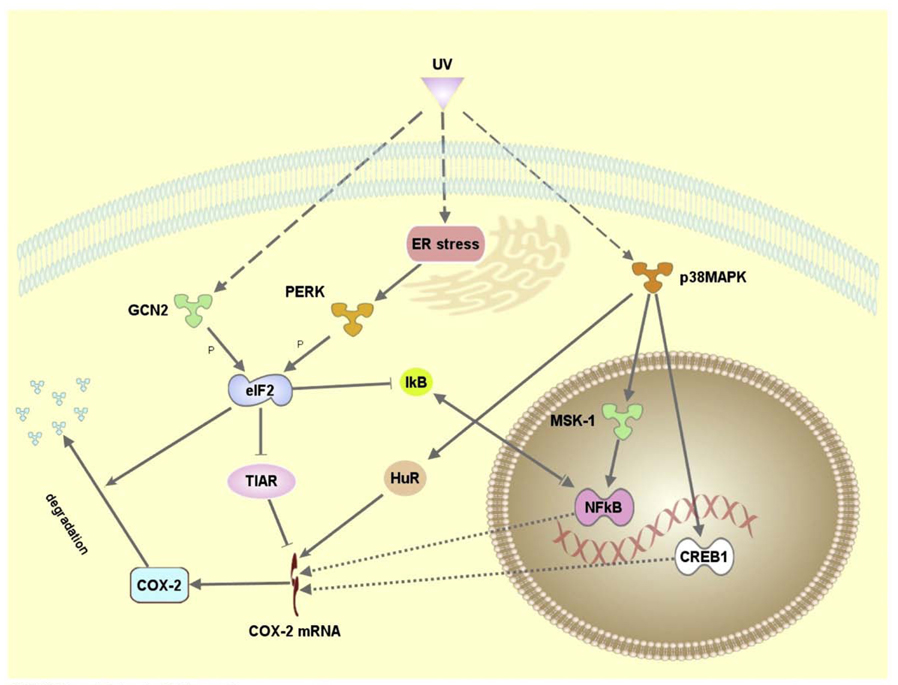
Conclusion
Our results indicate that the UVC-induced eIF2α phosphorylation-mediated translation inhibition plays a role in regulation of COX-2 expression via a complex mechanism at both transcriptional and translational levels. Using Ingenuity Pathways Analysis™ (Ingenuity Systems, Inc), we generated a description of a novel mechanism for the regulation of UVC-induced expression of COX-2 expression (Fig. 5).We propose that activation of eIF2α kinases leads to translational inhibition of IκB synthesis and activation of NF-κB, which in turn induces COX-2 transcription in the early stage of UVC-irradiation. The eIF2α phosphorylation has a two-tier effect. While it reduces the translation efficiency of COX-2 mRNA at both early and late stages of UVC-irradiation through its global translational inhibition, it also reduces the expression of COX-2 mRNA binding protein TIAR, thus promoting the translation of its target in the late stages of UVC-irradiation.
Fig. 5.
Proposed model for UVC induced COX-2 regulation. The molecular networks were generated by Ingenuity Pathway Analysis (Ingenuity® Systems) using Ingenuity database established on previously published data.
Acknowledgements
This work was supported by the National Institutes of Health Grant RO1 CA86926 (to SW), R56 CA086928 (to SW) and Egyptian Cultural and Educational Bureau (to SF).
References
- Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclooxygenase-2 gene. The Biochemical Journal. 1994;302(Pt 3):723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelor MA, Silvers AL, Bowden GT. The role of p38 in UVA-induced cyclooxygenase-2 expression in the human keratinocyte cell line, HaCaT. Oncogene. 2002;21:7092–7099. doi: 10.1038/sj.onc.1205855. [DOI] [PubMed] [Google Scholar]
- Bingham S, Beswick PJ, Blum DE, Gray NM, Chessell IP. The role of the cylooxygenase pathway in nociception and pain. Seminars in Cell and Developmental Biolology. 2006;17:544–554. doi: 10.1016/j.semcdb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. Journal of Immunology. 2000;164:3018–3025. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Research. 1999;59:991–994. [PubMed] [Google Scholar]
- Chen JJ, Huang WC, Chen CC. Transcriptional regulation of cyclooxygenase-2 in response to proteasome inhibitors involves reactive oxygen species-mediated signaling pathway and recruitment of CCAAT/enhancer-binding protein delta and CREB-binding protein. Molecular Biology of the Cell. 2005;16:5579–5591. doi: 10.1091/mbc.E05-08-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- Cok SJ, Morrison AR. The 3′-untranslated region of murine cyclooxygenase-2 contains multiple regulatory elements that alter message stability and translational efficiency. The Journal of Biological Chemistry. 2001;276:23179–23185. doi: 10.1074/jbc.M008461200. [DOI] [PubMed] [Google Scholar]
- Cok SJ, Acton SJ, Morrison AR. The proximal region of the 3′-untranslated region of cyclooxygenase-2 is recognized by a multimeric protein complex containing HuR, TIA-1, TIAR, and the heterogeneous nuclear ribonucleoprotein U. The Journal of Biological Chemistry. 2003;278:36157–36162. doi: 10.1074/jbc.M302547200. [DOI] [PubMed] [Google Scholar]
- Cok SJ, Acton SJ, Sexton AE, Morrison AR. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2mRNA. The Journal of Biological Chemistry. 2004;279:8196–8205. doi: 10.1074/jbc.M308475200. [DOI] [PubMed] [Google Scholar]
- Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. The Journal of rheumatology Supplement. 1997;49:15–19. [PubMed] [Google Scholar]
- Dean JL, Sarsfield SJ, Tsounakou E, Saklatvala J. p38 Mitogen-activated protein kinase stabilizes mRNAs that contain cyclooxygenase-2 and tumor necrosis factor AU-rich elements by inhibiting deadenylation. The Journal of Biological Chemistry. 2003;278:39470–39476. doi: 10.1074/jbc.M306345200. [DOI] [PubMed] [Google Scholar]
- Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N. Activation of GCN2 in UV-irradiated cells inhibits translation. Current Biology. 2002;12:1279–1286. doi: 10.1016/s0960-9822(02)01037-0. [DOI] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Molecular and Cellular Biology. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. The EMBO Journal. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald GA. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nature Reviews Drug Discovery. 2003;2:879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- Gustin JA, Pincheira R, Mayo LD, Ozes ON, Kessler KM, Baerwald MR, Korgaonkar CK, Donner DB. Tumor necrosis factor activates CRE-binding protein through a p38 MAPK/MSK1 signaling pathway in endothelial cells. American Journal of Physiology Cell Physiology. 2004;286:C547–C555. doi: 10.1152/ajpcell.00332.2002. [DOI] [PubMed] [Google Scholar]
- Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. European Journal of Biochemistry. 2003;270:3408–3421. doi: 10.1046/j.1432-1033.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- Harper KA, Tyson-Capper AJ. Complexity of COX-2 gene regulation. Biochemical Society Transactions. 2008;36:543–545. doi: 10.1042/BST0360543. [DOI] [PubMed] [Google Scholar]
- Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. The Journal of Biological Chemistry. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- Jang BC, Munoz-Najar U, Paik JH, Claffey K, Yoshida M, Hla T. Leptomycin B, an inhibitor of the nuclear export receptor CRM1, inhibits COX-2 expression. The Journal of Biological Chemistry. 2003;278:2773–2776. doi: 10.1074/jbc.C200620200. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Joseph K, Subramaniam K, Raymond JR, Tholanikunnel BG. Translational control of beta2-adrenergic receptor mRNA by T-cell-restricted intracellular antigen-related protein. The Journal of Biological Chemistry. 2005;280:1931–1943. doi: 10.1074/jbc.M405937200. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochemical Society Transactions. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P, Levidou G, Saetta AA, El-Habr E, Eftichiadis C, Demenagas P, Thymara I, Xiromeritis K, Boviatsis E, Thomas-Tsagli E, et al. Expression of nuclear factor-kappaB in human astrocytomas: relation to pI kappa Ba, vascular endothelial growth factor, Cox-2, microvascular characteristics, and survival. Human Pathology. 2008;39:1143–1152. doi: 10.1016/j.humpath.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Laszlo CF, Wu S. Mechanism of UV-induced ikappabalpha-independent activation of NF-kappaB. Photochemistry and Photobiology. 2008;84:1564–1568. doi: 10.1111/j.1751-1097.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. The European Journal of Neuroscience. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. The Journal of Biological Chemistry. 1990;265:10805–10808. [PubMed] [Google Scholar]
- Marnett LJ. Aspirin and related nonsteroidal anti-inflammatory drugs as chemopreventive agents against colon cancer. Preventive Medicine. 1995;24:103–106. doi: 10.1006/pmed.1995.1017. [DOI] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Galban S, Lopez de Silanes I, Martindale JL, Atasoy U, Keene JD, Gorospe M. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8354–8359. doi: 10.1073/pnas.1432104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Hayakawa H, Sakamoto H, Sugano K. Homeobox protein CDX2 reduces Cox-2 transcription by inactivating the DNA-binding capacity of nuclear factor-kappaB. The American Journal of Gastroenterology. 2007;42:719–729. doi: 10.1007/s00535-007-2088-y. [DOI] [PubMed] [Google Scholar]
- Nagy N, Shiroto K, Malik G, Huang CK, Gaestel M, Abdellatif M, Tosaki A, Maulik N, Das DK. Ischemic preconditioning involves dual cardio-protective axes with p38MAPK as upstream target. Journal of Molecular and Cellular Cardiology. 2007;42:981–990. doi: 10.1016/j.yjmcc.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. The EMBO Journal. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochimica et Biophysica Acta. 2000;1470:M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. The Journal of Biological Chemistry. 1994;269:11769–11775. [PubMed] [Google Scholar]
- Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochemistry and Photobiology. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends in Biochemical Sciences. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-kappaB mediate COX-2 expression in human airway myocytes. American Journal of Physiology Lung Cellular and Molecular Physiology. 2003;285:L1087–L1098. doi: 10.1152/ajplung.00409.2002. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. The Journal of Biological Chemistry. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual Review of Biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Takeda H, Sonoshita M, Oshima H, Sugihara K, Chulada PC, Langenbach R, Oshima M, Taketo MM. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Research. 2003;63:4872–4877. [PubMed] [Google Scholar]
- Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins & Other Lipid Mediators. 2002:68–69. 95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Tang Q, Chen W, Gonzales MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene. 2001;20:5164–5172. doi: 10.1038/sj.onc.1204667. [DOI] [PubMed] [Google Scholar]
- Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Molecular and Cellular Biology. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. The International Journal of Biochemistry & Cell Biology. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Ulivi V, Giannoni P, Gentili C, Cancedda R, Descalzi F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. Journal of Cellular Biochemistry. 2008;104:1393–1406. doi: 10.1002/jcb.21717. [DOI] [PubMed] [Google Scholar]
- Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Molecular Carcinogenesis. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Molecular and Cellular Biology. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death and Differentiation. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Wu S, Hu Y, Wang JL, Chatterjee M, Shi Y, Kaufman RJ. Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. The Journal of Biological Chemistry. 2002;277:18077–18083. doi: 10.1074/jbc.M110164200. [DOI] [PubMed] [Google Scholar]
- Wu S, Tan M, Hu Y, Wang JL, Scheuner D, Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. The Journal of Biological Chemistry. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. The Journal of Clinical Endocrinology and Metabolism. 2005;90:286–295. doi: 10.1210/jc.2004-1612. [DOI] [PubMed] [Google Scholar]