Abstract
The objective of this study was to compare the health-related quality of life (HRQOL) of long-term to short-term high-grade glioma (HGG) survivors, determine the prognostic value of HRQOL for overall survival, and determine the effect of tumor recurrence on HRQOL for long-term survivors. Following baseline assessment (after surgery, before radiotherapy), self-perceived HRQOL (using the Medical Outcomes Study Short Form 36 [SF-36]) and brain tumor–specific symptoms (using the 20-item Brain Cancer Module) were assessed every 4 months until 16 months after histological diagnosis. Kaplan-Meier survival analysis and the Cox proportional hazards model were performed to estimate overall survival of patients with impaired scores on the aggregated SF-36 higher-order summary scores measuring physical functioning on a physical component scale and on a mental component scale (MCS). Sixteen patients with a short-term survival (baseline and 4-month follow-up) and 16 with a long-term survival (follow-up until 16 months after diagnosis) were selected out of 68 initially recruited HGG patients. At baseline, the short-term and long-term survivors did not differ in their HRQOL. Between baseline and the 4-month follow-up, HRQOL of short-term survivors deteriorated, whereas the long-term survivors improved to a level comparable to healthy controls. Patients with impaired mental functioning (MCS) at baseline had a shorter median survival than patients with normal functioning. After accounting for differences in patient and tumor characteristics, however, mental functioning was not independently related to poorer overall survival. Not surprisingly, in the group of long-term survivors, the five patients with recurrence had a more compromised HRQOL at the 16-month follow-up compared to the 11 patients without recurrence. We concluded that baseline HRQOL is not related to duration of survival and that long-term survivors show improvement of HRQOL to a level comparable to that of the healthy.
Keywords: health-related quality of life, high-grade glioma survivors
High-grade gliomas (HGGs) are the most frequent primary brain tumors, with an annual incidence of 3–4 per 100,000.1 Within the group of HGGs, glioblastoma multiforme (GBM) is by far the most common and most aggressive subtype. Although treatment modalities have been refined over the years (microsurgery, radiotherapy, and concomitant chemotherapy), the overall prognosis in these patients remains dismal. While significant progress has been achieved with the introduction of radiotherapy plus concomitant and adjuvant temozolomide for GBM patients,2 the prognosis of the majority of HGG patients is still predominantly correlated with nonmodifiable factors such as age, tumor grade, and performance status at the time of diagnosis.3–7
During the last decades, many studies evaluating new treatment protocols for cancer patients mainly focused on overall survival and progression-free survival (PFS) as primary response measures. It is increasingly recognized that the choice of treatment also should entail careful consideration of its effects on the health-related quality of life (HRQOL) during the remaining survival time. Considering their limited survival, this is even more urgent in patients with HGGs. As survival is limited, patients optimally should be informed of the impact of all treatment options on their quality of life at the time of diagnosis. Relatively little is known about HRQOL during the disease course of patients with HGGs. Although one might intuitively expect a decrease in HRQOL in the course of the disease, this does not necessarily have to be the case. A recent European Organization for Research and Treatment of Cancer (EORTC) study showed that newly diagnosed GBM patients did not report lasting negative effects of tumor treatment with radiotherapy plus concomitant and adjuvant temozolomide on HRQOL and even demonstrated a slight improvement after diagnosis, until 6 months after they started the actual treatment.8 The authors attributed this improvement to the limited number of patients with recurrent disease during the first 6 months and speculated that the improvement could be the result of so-called survivorship.
Indices of HRQOL may serve as independent predictors of survival and can thus be very useful in clinical practice when treatment decisions have to be made. Only a few studies have addressed this issue in patients with primary brain tumors. Sehlen and colleagues,9 for instance, showed that HRQOL as measured by the FACT-G total score, and not other patient and tumor characteristics, was predictive of survival in a patient population with primary and secondary brain tumors. However, dissimilar results were obtained from the study of Mauer et al.10 who evaluated the predictive value of HRQOL following surgery in newly diagnosed brain tumor patients treated with either radiotherapy alone or radiotherapy and concomitant temozolomide followed by six adjuvant cycles of temozolomide. They found HRQOL to have only little predictive value in addition to established clinical factors such as age, performance status, extent of surgery, corticosteroids at entry, cognitive status, and MGMT promotor methylation status. These contradictory findings appear to be mainly due to differences in statistical analyses. The aforementioned lack of information on the course of HRQOL during treatment, as well as the contradictory results from previous studies on the predictive value of HRQOL, clearly demonstrate the necessity to employ further research into the prognostic value of HRQOL. In this article we describe the course of HRQOL of a heterogeneous cohort of patients with HGG. In particular, we compared initial HRQOL of those patients still alive at least 2 years following diagnosis (i.e., long-term survivors) with initial HRQOL of those who died within 1 year after the histological diagnosis (i.e., short-term survivors) and evaluated the HRQOL at different follow-up moments to determine when improvement or deterioration is to be expected in the course of the disease. Furthermore, we evaluated the prognostic value of HRQOL in this patient population and the differences in HRQOL between long-term survivors with and without tumor progression in the course of their disease.
Patients and Methods
Patients
This study is part of a large nationwide longitudinal study in the Netherlands into the neurocognitive status and HRQOL of high- and low-grade glioma patients and their partners. The results of these patients’ neurocognitive function and HRQOL at baseline (i.e., following surgery, prior to the start of radiotherapy) have been published previously.11 In short, consecutive, newly diagnosed, histologically confirmed patients were recruited from six centers in the Netherlands (listed in Acknowledgments) based on the following criteria: (1) had WHO grade III astrocytoma, oligodendroglioma. or oligoastrocytoma, or WHO grade IV glioma (GBM); (2) had an estimated life expectancy of at least 3 months; (3) were eligible for radiotherapy; and (4) were able to communicate in the Dutch language. The institutional review boards of all participating hospitals approved the study protocol. Patients were asked to participate by their treating physician (i.e., neurologist, neurosurgeon, medical oncologist, or radiation oncologist), and informed consent was obtained. Medical charts were reviewed to obtain data on patient, tumor, and treatment characteristics. Patients were asked to fill out the questionnaires listed below, at baseline and every 4 months thereafter until 16 months after histological diagnosis.
Outcome Measures
Health-related quality of life
The Medical Outcomes Study Short Form 36 (SF-36) is a self-report questionnaire composed of 36 items, organized into eight multi-item scales assessing physical functioning (PF), role limitations caused by physical functioning (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations caused by emotional problems (RE), and general mental health (MH). Raw scores were converted linearly to 0–100 scale scores, with higher scores indicating better functioning.12 In addition to individual SF-36 scale scores, higher-order component scores were calculated on two scales: a physical component scale (PCS) and a mental component scale (MCS).
Brain tumor–specific symptoms
Brain tumor–specific symptoms were assessed by means of a questionnaire (20-item Brain Cancer Module [BCM-20]).13 The BCM-20 contains five multi-item scales consisting of future uncertainty, visual disorder, motor dysfunction, communication deficit, and emotional distress. It also contains seven single items (headache, seizures, drowsiness, hair loss, itching, weakness of legs, and difficulties with bladder control). In this study we did not include the emotional distress item because of the overlap with the SF-36 mental health scale. We also did not include hair loss, itching, and bladder control. Raw scores of the BCM-20 were linearly converted to 0–100 scale scores, with higher scores representing lower levels of functioning.
Statistical analysis
Short-term survivors were defined as those patients out of the 68 HGG patients initially included who had a baseline and 4-month follow-up assessment and who died within 1 year following the histological diagnosis. Following baseline, long-term survivors had a follow-up at 4, 8, 12, and 16 months and survived for over 2 years. Pearson’s chi-square test or analysis of covariance (ANCOVA), correcting for differences in age, sex, and marital status, was used to test for differences between patient groups in their self-perceived HRQOL. The nonparametric Wilcoxon signed-rank test was used to test for significance of potential changes in HRQOL of HGG patients between baseline and the subsequent follow-ups.
For the analysis of the predictive value of baseline HRQOL scores on overall survival, we analyzed data of all 68 patients of whom baseline performance has been reported elsewhere.11 Kaplan-Meier analysis was performed to obtain estimates of overall survival of patients with an impaired score on the SF-36 PCS and MCS disregarding differences in tumor, treatment, or patient characteristics. Subsequently, the Cox proportional hazards model was used to assess the associations between overall survival on the one hand and MCS and PCS scores above or beneath the median score of the whole group of 68 patients accounting for age, tumor grade, extent of surgery, tumor lateralization, and performance status on the other.
To gauge the influence of tumor recurrence on HRQOL at 16-month follow-up, the long-term survivors were stratified by the presence or absence of tumor recurrence during their follow-up. Subsequently, non-parametric Mann-Whitney U tests were used to test for differences between the two patient groups. All statistical analyses employed were two-tailed with alpha set at 0.05.
Results
Patient Characteristics
Eighteen of the 90 eligible HGG patients declined to participate because they expected the study to be too stressful, and four patients were excluded because of severe aphasia. Consequently, 68 HGG patients were included in the study. Of these 68 patients, 16 died within 1 year after histological diagnosis (i.e., short-term survivors) and 16 were still alive 2 years after the histological diagnosis (i.e., long-term survivors). The other 36 patients died between 1 and 2 years after diagnosis. Table 1 shows the characteristics of these patients stratified by survival. As expected, long-term survivors were significantly younger (p = 0.001) and more often had less malignant (WHO grade III) tumors (p = 0.000). However, the functional status at baseline (i.e., KPS scores) did not vary between the long- and short-term survivors.
Table 1.
Patient characteristics at baseline of short-term (deceased within 1 year after histological diagnosis) and long-term survivors (still alive 2 years after histological diagnosis)
| Short-Term Survivors (n = 16) | Long-Term Survivors (n = 16) | p Value | |
|---|---|---|---|
| Age ± SD (years) | 56.4 ± 11.8 | 40.9 ± 13.2 | 0.001 |
| Sex: male/female (%) | 100/0 | 75/25 | 0.033 |
| Marital status: married/unmarried (%) | 100/0 | 50/50 | 0.001 |
| Education: mean/SD | 4.1/1.7 | 4.3/2.0 | 0.179 |
| Tumor grade: III/IV (%) | 0/100 | 62.5/37.5 | 0.000 |
| Tumor lateralization: left/right/bilateral (%) | 31.3/68.7/0 | 50/50/0 | 0.280 |
| Neurosurgery: biopsy/resection (%) | 0/100 | 6.3/93.7 | 0.310 |
| Total dose radiotherapy (Gy): mean/range/SD | 49/28–64/10.8 | 57/42–66/7.5 | 0.210 |
| Number of fractions radiotherapy: mean/range/SD | 20/4–32/9.6 | 28/12–33/7.1 | 0.032 |
| KPS: mean/range | 80/50–100 | 80/60–100 | 0.616 |
| Antiepileptic drug use: yes/no/unknown (%) | 75/18.8/6.2 | 68.8/31.3/0 | 0.462 |
| Dexamethasone use: yes/no/unknown (%) | 31.2/43.8/25 | 25/75/0 | 0.066 |
| Survival (months): mean/range/SD | 9.8/5–12/2.4 | 38.3/27–59/11.7 | 0.000 |
Since only three short-term survivors had a follow-up at 8 months, we decided to evaluate baseline and 4-month follow-up. All long-term survivors had a follow-up until 16 months after the histological diagnosis. During follow-up, 5 of the 16 long-term survivors were confronted with tumor recurrence.
At baseline, 23 of 32 survivors were on mono- or polytherapy with the older antiepileptic drugs such as valproic acid, carbamazepine, and phenytoin. Between baseline and 4-month follow-up, the short-term survivors only received radiotherapy. During follow-up, however, 7 of the 16 sixteen long-term survivors received subsequent antitumor treatment after the initial radiotherapy. Three of the 11 patients without tumor progression during follow-up received treatment. One patient received six cycles of PCV (procarbazine, CCNU [lomustine], and vincristine) in the adjuvant setting, one patient underwent stereotactic radiotherapy 3 months after the initial conventional radiotherapy, and a third patient received dibromodulocitol and BCNU (carmustine) as part of a trial.
Four of the five long-term survivor patients with tumor progression during follow-up received treatment: two patients were subsequently treated with four cycles of PCV, and one of these two patients again underwent radiotherapy (13 × 3 Gy). One patient received two cycles of PCV and the fourth patient received stereotactic radiotherapy (1 × 15 Gy).
HRQOL of Short-Term and Long-Term Survivors
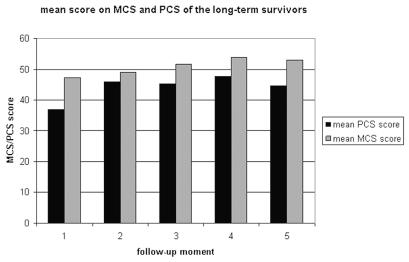
Mean SF-36 scores of short-term survivors at baseline and 4-month follow-up are displayed in Table 2. Mean SF-36 scores of long-term survivors at baseline and 4-, 8-, 12-, and 16-month follow-ups are displayed in Table 3. During the course of their disease, the short-term survivors developed more general health problems (Wilcoxon; p = 0.015), while HRQOL in the other domains remained unaffected. The long-term survivors, on the other hand, experienced improvement in physical, social, and emotional functioning in the course of their disease. These improvements were also reflected in the physical higher-order component scales (PCS) in long-term survivors. The scores on the PCS and MCS of the long-term survivors are displayed in Fig. 1.
Table 2.
SF-36 scores of short-term survivors at baseline and 4-month follow-up
| Baseline | 4-month Follow-up | p Valuea | |
|---|---|---|---|
| Scale | Mean (SD) | Mean (SD) | |
| PF | 63.1 (28.6) | 53.9 (32.4) | NS |
| RP | 14.1 (24.1) | 21.7 (38.8) | NS |
| BP | 65.4 (28.9) | 78.6 (20.4) | NS |
| GH | 56.5 (19.2) | 39.7 (17.2) | 0.015 |
| VT | 59.0 (20.0) | 47.8 (21.9) | NS |
| SF | 67.2 (25.0) | 70.0 (31.6) | NS |
| RE | 48.9 (45.2) | 42.2 (42.7) | NS |
| MH | 67.8 (19.4) | 63.5 (21.5) | NS |
Abbreviations: SF-36, Medical Outcomes Study Short Form 36; PF, physical functioning; NS, not significant; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Nonparametric (Wilcoxon).
Table 3.
SF-36 scores of long-term survivors at baseline and 4-, 8-, 12-, and 16-month follow-up
| Scale | Baseline Mean (SD) | 4-Month Follow-up Mean (SD) | 8-Month Follow-up Mean (SD) | 12-Month Follow-up Mean (SD) | 16-Month Follow-up Mean (SD) |
|---|---|---|---|---|---|
| PF | 65.2 (24.1) | 79.4a (18.5) | 75.9 (24.1) | 82.3b (20.1) | 78.8c (20.7) |
| RP | 21.9 (35.2) | 46.4 (40.3) | 54.7d (45.8) | 67.9e (42.1) | 61.0f (43.8) |
| BP | 57.3 (25.8) | 87.2g (16.4) | 85.9h (20.0) | 90.9i (12.6) | 81.3j (24.2) |
| GH | 53.4 (18.1) | 59.4 (18.6) | 58.9 (20.3) | 62.3 (18.6) | 55.3 (19.1) |
| VT | 57.5 (21.4) | 58.2 (24.6) | 66.0 (20.8) | 68.6 (13.4) | 65.3 (19.1) |
| SF | 55.5 (33.8) | 75.9 (22.7) | 76.6k (27.7) | 80.8l (18.2) | 82.8m (17.0) |
| RE | 62.5 (40.1) | 76.2 (33.2) | 75.0 (41.3) | 92.9n (26.7) | 87.5 (34.2) |
| MH | 73.5 (12.0) | 73.1 (18.5) | 79.8 (16.3) | 80.9 (22.7) | 78.0 (13.8) |
Abbreviations: SF-36, Medical Outcomes Study Short Form 36; PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Improvement in physical functioning between baseline and 4-month follow-up (nonparametric Wilcoxon; p = 0.036).
Improvement in physical functioning between baseline and 12-month follow-up (p = 0.017).
Improvement in physical functioning between baseline and 16-month follow-up (p = 0.012).
Improvement in physical role between baseline and 8-month follow-up (p = 0.012).
Improvement in physical role between baseline and 12-month follow-up (p = 0.007).
Improvement in physical role between baseline and 16-month follow-up (p = 0.027).
Improvement in complaints of bodily pain between baseline and 4-month follow-up (p = 0.010).
Improvement in complaints of bodily pain between baseline and 8-month follow-up (p = 0.002).
Improvement in complaints of bodily pain between baseline and 12-month follow-up (p = 0.017).
Improvement in complaints of bodily pain between baseline and 16-month follow-up (p = 0.027).
Improvement in social functioning between baseline and 8-month follow-up (p = 0.029).
Improvement in social functioning between baseline and 12-month follow-up (p = 0.029).
Improvement in social functioning between baseline and 16-month follow-up (p = 0.007).
Improvement in emotional role between baseline and 12-month follow-up (p = 0.005).
Fig. 1.
Mean physical component scale (PCS) and mental component scale (MCS) scores of long-term survivors at the different follow-up moments. (1 = baseline; 2 = 4-month; 3 = 8-month; 4 = 12-month; 5 = 16-month).
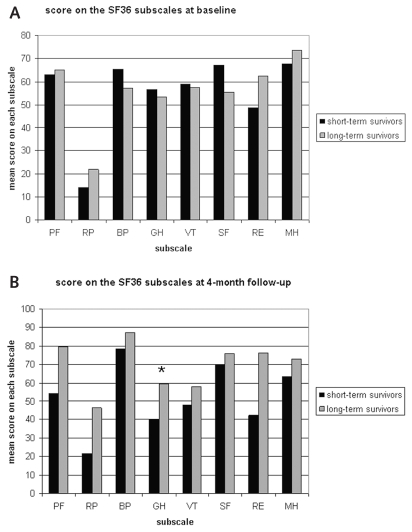
Comparison of the HRQOL of the long- and short-term survivors (see Fig. 2) showed that long-term survivors reported fewer general health problems (ANCOVA; p = 0.027) at 4-month follow-up than did short-term survivors. Comparison of the two higher-order component scores showed a significantly lower PCS in the short-term survivors at 4-month follow-up (ANCOVA; p = 0.034) when compared to the long-term survivors. No significant differences were found in mental functioning as expressed by the MCS.
Fig. 2.
Medical Outcomes Study Short Form 36 (SF-36) scores of short- and long-term survivors at baseline (A) and SF-36 scores of the short- and long-term survivors at 4-month follow-up (B). Abbreviations: PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Brain Tumor– and Treatment-Specific Symptoms of Short-Term and Long-Term Survivors
Table 4 reports the mean scores for the single items of the BCM-20 in short-term survivors. Between baseline and 4-month follow-up, short-term survivors showed an increased weakness of legs (Wilcoxon; p = 0.026). Symptoms of long-term survivors did not change within this time frame (Table 5). Comparison of the BCM-20 of the long- and short-term survivors showed that long-term survivors reported less weakness of legs (ANCOVA; p = 0.027) at the 4-month follow-up when compared to short-term survivors. Table 5 reports the mean scores for the single items of the BCM-20 in long-term survivors. Symptoms in these patients were relatively stable over time. The long-term survivors suffered significantly less from future uncertainty at the 8-, 12-, and 16-month follow-ups compared to their baseline evaluation (Wilcoxon; p = 0.041, p = 0.010, and p = 0.002, respectively). Furthermore, the long-term survivors also had fewer headaches and less weakness of legs at 12-month follow-up compared to the baseline assessment (Wilcoxon; p = 0.047 and p = 0.046, respectively).
Table 4.
Mean scores of the single items of the BCM-20 of short-term survivors
| Symptom | Baseline Mean (SD) | 4-Month Follow-up Mean (SD) | p Valuea Mean |
|---|---|---|---|
| Future uncertainty | 51.4 (26.9) | 51.1 (28.0) | NS |
| Visual disorder | 20.8 (20.2) | 15.6 (12.5) | NS |
| Motor dysfunction | 19.4 (20.1) | 33.0 (34.8) | NS |
| Communication deficit | 20.1 (30.4) | 25.2 (32.1) | NS |
| Headache | 35.4 (37.5) | 24.4 (29.5) | NS |
| Seizures | 12.5 (26.9) | 26.7 (31.4) | NS |
| Drowsiness | 22.9 (23.5) | 26.2 (23.3) | NS |
| Leg weakness | 12.5 (20.6) | 35.6 (32.0) | 0.026 |
Abbreviations: BCM-20, 20-item Brain Cancer Module; NS, not significant.
Nonparametric (Wilcoxon).
Table 5.
Mean scores of the single items of the BCM-20 of long-term survivors
| Symptom | Baseline Mean (SD) | 4-Month Follow-up Mean (SD) | 8-Month Follow-up Mean (SD) | 12-Month Follow-up Mean (SD) | 16-Month Follow-up Mean (SD) |
|---|---|---|---|---|---|
| Future uncertainty | 43.8 (30.5) | 31.6 (28.0) | 25.0a (20.4) | 22.0b (23.1) | 19.1c (17.8) |
| Visual disorder | 12.5 (14.6) | 18.3 (23.3) | 11.1 (14.1) | 10.4 (18.5) | 12.2 (15.2) |
| Motor dysfunction | 15.3 (14.0) | 8.7 (13.2) | 14.6 (17.1) | 10.7 (15.8) | 15.3 (17.2) |
| Communication deficit | 20.8 (16.2) | 18.3 (19.8) | 18.8 (20.2) | 23.7 (21.8) | 22.2 (23.0) |
| Headache | 31.3 (33.3) | 11.9 (16.6) | 18.8 (24.3) | 8.9d (15.3) | 16.7 (21.1) |
| Seizures | 8.3 (14.9) | 14.2 (21.5) | 10.4 (20.1) | 4.4 (11.7) | 16.7 (29.8) |
| Drowsiness | 15.6 (21.3) | 19.1 (31.3) | 10.4 (20.1) | 13.3 (21.8) | 10.4 (23.5) |
| Leg weakness | 8.3 (15.0) | 0 (0) | 6.3 (18.1) | 0e (0) | 14.6 (29.7) |
Abbreviation: BCM-20, 20-item Brain Cancer Module.
Significant improvement between baseline and 4-month follow-up (nonparametric Wilcoxon; p = 0.041).
Improvement in future uncertainty between baseline and 12-month follow-up (p = 0.010).
Improvement in future uncertainty between baseline and 16-month follow-up (p = 0.002).
Improvement in headache complaint between baseline and 12-month follow-up (p = 0.047).
Improvement in leg weakness between baseline and 12-month follow-up (p = 0.046).
Prognostic Value of Baseline HRQOL on Survival
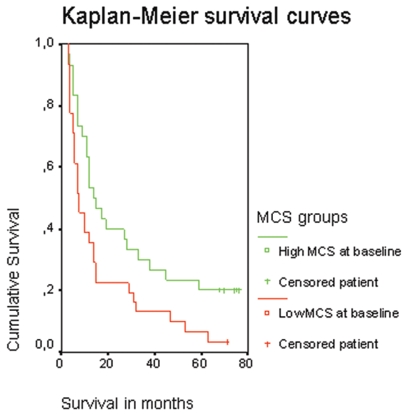
Of the 68 patients included in the study, 62 had died at the time of statistical analysis; thus, data of six patients were censored. Kaplan-Meier analysis yielded significant differences in the median survival between patients with a baseline MCS score lower than the median (median MCS score = 45, n = 31), and those patients with a MCS score higher than median at baseline (n = 31). Patients with a low MCS score at baseline had a significantly shorter median survival (8 months) than those with a high MCS score (14.5 months; log rank test: p = 0.023) as shown in Fig. 3.
Fig. 3.
Kaplan-Meier survival curves of patient groups stratified by the mental component scale (MCS) score at baseline.
However, a multivariate Cox proportional hazards model of survival time, including score on the MCS, age, sex, tumor grade, tumor treatment, and KPS, showed that baseline MCS score did not significantly add prognostic value to the model including these patient and tumor characteristics.
Influence of Tumor Recurrence on HRQOL and Symptoms in Long-Term Survivors
Because we expected tumor recurrence to influence the HRQOL and brain tumor–specific symptoms in long-term survivors, we evaluated the HRQOL and brain tumor–specific symptoms of the 16 long-term survivors at the 16-month follow-up. Five of the 16 patients suffered from tumor recurrence during follow-up: two patients at 4 months (one of them was subsequently treated with four cycles of PCV, and one patient again underwent radiotherapy [13 × 3 Gy]). At 8-month follow-up another two patients demonstrated tumor recurrence (one patient received two cycles of PCV, and one patient received no treatment), and one more patient suffered from tumor recurrence between the 12- and 16-month follow-ups and received stereotactic radiotherapy (1 × 15 Gy).
Patients with tumor recurrence during follow-up evidently had more problems with physical functioning (Mann-Whitney U; p = 0.000), with work or other daily activities resulting from physical health limitations (p = 0.003), mental health (p = 0.027), and general health (p = 0.019) at 16-month follow-up than those without recurrence. The increase in physical complaints was also reflected in the higher-order physical component scale (PCS; p = 0.003). Long-term survivors without tumor recurrence experienced significantly less motor dysfunction (Mann-Whitney U; p = 0.002), weakness of legs (p = 0.009), visual disorders (p = 0.013), and future uncertainty (p = 0.027) than long-term survivors with tumor recurrence.
Discussion
In this study we evaluated both HRQOL and brain tumor–specific symptoms among HGG patients in the course of their disease. Our primary objective was to investigate whether long- and short-term survivors of high-grade gliomas were characterized by different HRQOL and symptom patterns. Our second goal was to evaluate the prognostic value of baseline HRQOL on overall survival, and our third goal was to determine whether the HRQOL of long-term survivors with tumor recurrence during our follow-up differs from HRQOL of patients without recurrence during follow-up.
Following surgery, and prior to radiotherapy, long-term and short-term survivors did not differ in HRQOL. From baseline until the 4-month follow-up, however, short-term survivors reported a more compromised HRQOL. In contrast with short-term survivors, the long-term survivor group showed improvements in HRQOL during the course of their disease with further improvement in physical functioning at each consecutive follow-up moment. Most surprising, physical functioning of these long-term survivors as expressed by PCS scores even reached levels comparable to that of a healthy control population.14
The physical improvements observed in the long-term survivors during the first months after inclusion can be attributed to the recuperation from the surgery and radiotherapy, but this factor cannot fully explain the subsequent improvements in later stages of the disease. The long-term survivors, although confronted with a brain tumor with an expected poor prognosis, complained less about future uncertainty between the 8- and 16-month follow-up, which might be attributed to the fact that patients at that time realize that they are functioning relatively well after surgery and radiotherapy (the so-called “survivorship” feeling).
Salander and colleagues15 interviewed 30 patients confronted with the diagnosis of a malignant brain tumor. They showed that most patients were aware of the fact that the tumor exposed them to grave danger, but that they were able to use various cognitive maneuvers at the same time to create protection and hope. Another explanation for the counterintuitive findings relates to the so-called response shift. An adaptive psychological process may account for the poor correlation between disease severity, treatment burden, and prognosis on the one hand, and the HRQOL appraisal on the other. It is possible that patients may progressively narrow their focus on the pursuit of certain goals, while disengaging from others. It is then possible to maintain a stable appraisal of the HRQOL by adjusting what they consider to be relevant to it.16,17 This may have been the case in the long-term survivors in our study.
In our study, mental functioning as measured by the MCS did not have value in predicting overall survival in addition to age, tumor grade, extent of surgery, tumor lateralization, and performance status. Our results corroborate those of Mauer et al.,10 who showed that the HRQOL and tumor-related symptoms, as measured through the 30-question EORTC Quality of Life Questionnaire (EORTC-QLQ-C30) and BCM-20, added relatively little to clinical factors that predict survival in newly diagnosed glioblastoma patients. Our data concur with those of Sehlen et al.,9 who showed that the FACT-G total score was an independent predictor of survival in patients with primary and secondary brain tumors. The differences between these studies could be induced by differences in patient population (Sehlen et al. evaluated both patients with a primary and secondary brain tumor, whereas Mauer et al. and we evaluated only patients with a high-grade primary brain tumor).
The long-term survivor group consisted of patients who suffered from tumor recurrence during the study and patients in whom the tumor recurred after the follow-up period. As expected, comparison of these two groups showed that the patients with recurrence had a more compromised functioning as expressed by PCS scores and complained more of motor dysfunction, weakness of legs, visual disorders, and future uncertainty. Giovagnoli et al.18 also showed that the global quality of life was more compromised in a group of patients with tumor recurrence at various time points compared to patients with stable disease. They showed that HRQOL was determined by multiple factors. In these patients, psychosocial factors were the strongest determinants of the HRQOL.18,19
The lack of difference in the score on the MCS between patients with and without tumor recurrence in our study suggests that patients were mentally stable despite the fact that they suffered from tumor recurrence and despite their physical problems. It is possible that this effect is a result of the aforementioned survivorship feeling and/or response shift.
Evidently, this study has its limitations. First of all, the patient group is rather small. To compare the long-term with short-term survivors, we were able to select 32 out of 68 HGG patients. Second, patients were included before the introduction of the combined treatment modality (radiotherapy with concomitant chemotherapy followed by six cycles of adjuvant temozolomide) for glioblastoma patients. However, we included not only glioblastoma, but also WHO grade III tumors, whose standard postoperative treatment nowadays still consists of radiotherapy. More important, only 37% of the population in this study were glioblastoma patients. Third, during follow-up, 7 of the 16 long-term survivors received subsequent antitumor treatment after the initial radiotherapy. Three of the 11 patients without tumor progression during follow-up received adjuvant treatment. Due to the small sample size and the variety of adjuvant antitumor treatment, no post hoc analysis was done stratifying for the different antitumor treatments.
In conclusion, long-term survivors show improvement in HRQOL during the course of their disease and even attain levels that are comparable to that of healthy controls, whereas short-term survivors start at a lower level and hardly show improvement. Furthermore, although better mental functioning following surgery predicts longer survival, it does not independently add to the predictive value of established patient and tumor characteristics. In the long-term survivor group, tumor recurrence interfered with HRQOL, particularly with physical problems and feelings of future uncertainty.
From this study we conclude that those patients with a long-term survival can have a good quality of life after the histological diagnosis, which is an important issue for both patient and caregiver and for the treating physician who has to consider treatment decisions carefully during the remaining survival time.
Acknowledgments
We thank J.C. Baaijen, J.S.A. Belderbos, E.B. Bongartz, W. Boogerd, D. González, M.C.C.M. Hulshof, J.A. Langendijk, I. van der Lee, S. Leenstra, W.F. Luitjes, J.G. Salverda, S.I. Tjahja, and C.A.F. Tulleken for permission to recruit their patients. HGG patients were recruited from the University Medical Center Utrecht; the Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital; VU University Medical Center; the Slotervaart Hospital; and the Academic Medical Center of the University of Amsterdam. This study was supported by grant no. VU96-1155 from the Dutch Cancer Society and by Kapteijnfonds.
References
- 1.Radhakrishnan K, Mokri B, Parisi JE, et al. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37:67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Aiken RD. Quality-of-life issues in patients with malignant gliomas. Semin Oncol. 1994;21:273–275. [PubMed] [Google Scholar]
- 4.Taylor BV, Buckner JC, Cascino TL, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol. 1998;16:2195–2201. doi: 10.1200/JCO.1998.16.6.2195. [DOI] [PubMed] [Google Scholar]
- 5.Meyers CA, Hess KR, Yung WK, et al. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 6.Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? . Neurooncol. 2003;5:255–260. doi: 10.1215/S1152851703000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61:1796–1798. doi: 10.1212/01.wnl.0000098892.33018.4c. [DOI] [PubMed] [Google Scholar]
- 8.Taphoorn MJB, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 9.Sehlen S, Lenk M, Hollenhorst H, et al. Quality of life (QoL) as a predictive mediator variable for survival in patients with intracerebral neoplasma during radiotherapy. Onkologie. 2003;26:38–43. doi: 10.1159/000069862. [DOI] [PubMed] [Google Scholar]
- 10.Mauer M, Stupp R, Taphoorn MJB, et al. The prognostic value of health-related quality-of-life data in predicting survival in glioblastoma cancer patients: results from an international randomised phase III EORTC Brain Tumour and Radiation Oncology Groups, and NCIC Clinical Trials Group study. Br J Cancer. 2007;97:302–307. doi: 10.1038/sj.bjc.6603876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19:4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Sherbourne CD. MOS-36–item short form healthy survey (SF-36): I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 13.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Sherbourne CD. Santa Monica, CA: Rand Corporation; 1992. RAND 36-Item Health Survey 1.0 Scoring Manual. [Google Scholar]
- 15.Salander P, Bergenheim T, Henriksson R. The creation of protection and hope in patients with malignant brain tumours. Soc Sci Med. 1996;42:985–996. doi: 10.1016/0277-9536(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz CE, Rapkin BD. Reconsidering the psychometrics of quality of life assessment in light of response shift. Health Qual Life Outcomes. 2004;2:16. doi: 10.1186/1477-7525-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapkin BD, Schwartz CE. Towards a theoretical model of quality-of-life appraisal: implications of findings from studies of response shift. Health Qual Life Outcomes. 2004;2:14. doi: 10.1186/1477-7525-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76:562–568. doi: 10.1136/jnnp.2004.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovagnoli AR. Quality of life in patients with stable disease after surgery, radiotherapy, and chemotherapy for malignant brain tumour. J Neurol Neurosurg Psychiatry. 1999;67:358–363. doi: 10.1136/jnnp.67.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]