Abstract
Purpose
To compare closed-system solid surface vitrification with slow freezing.
Methods
Mouse 2-cell embryos (n = 348) were divided into vitrification, slow freezing and non-frozen groups. For vitrification, embryos were exposed to 10% ethylene glycol (EG), 10% dimethylsulfoxide (DMSO) and 10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS) for 10 min, then transferred into 17.5% EG, 17.5% DMSO, 0.25 M trehalose and 10% FBS in PBS. They were placed on hemi-straws and inserted into 0.5 ml straws inside a previously cooled aluminum cylinder. Slow freezing was done in straws by the conventional method.
Results
Vitrified embryos had significantly higher survival, further cleavage and blastocyst formation rates than those in the slow freezing group (p < 0.001) and were comparable to controls. Blastocysts in the vitrification and control groups had significantly more cells than those in the slow freezing group (p < 0.05).
Conclusions
Closed-system vitrification was more effective than conventional slow freezing.
Keywords: Embryo cryopreservation, Slow freezing, Solid surface vitrification, Total cell number
Introduction
Slow programmable freezing is currently the standard method of embryo cryopreservation in most assisted conception centers world-wide [1]. However, the method requires an expensive biological freezer and the process is time-consuming. A fast alternative method, without the use of costly equipment, would provide significant benefits, especially to assisted conception centers in developing countries. In this regard, vitrification stands out as an attractive alternative to slow cooling. In fact, some researchers predict that slow freezing will be replaced entirely by the new vitrification techniques sooner or later in all relevant areas of gametes and embryo freezing [2].
The new vitrification techniques require the use of minimal freezing volume and an open container (or container-less techniques) to achieve a very rapid cooling rate. Popular containers that allow a direct contact between the embryos and liquid nitrogen, or a cold metal surface, include electron microscope grids [3], cryoloops [4], open-pulled straws [5], flexipette [6], Cryotop [7] or CryoTip techniques [8]. These methods, however, pose a potential risk of disease transmission through contaminated liquid nitrogen or metal surface during cooling and storage [9].
We have designed a closed vitrification system, which is inexpensive and has the potential for future use in human embryo cryopreservation. In this study, we used mouse embryos as a model to compare the efficacy of our in-house method of vitrification with that of the standard slow programmable freezing.
Materials and methods
Outbred ICR (Institute Cancer Research) mice were purchased form the National Animal Institute, Mahidol University, Bangkok, Thailand. They were kept at the Animal Husbandry Unit, Faculty of Medicine, Chiang Mai University, in a well-ventilated room at 25 ± 2°C, under 60–70% humidity and controlled 12 h light/12 h dark cycles. We closely followed international and national guidelines for ethical conduct in the care and use of animals for research. The Animal Ethics Committees (AECs) of the Faculty of Medicine, Chiang Mai University approved the use of mice in our study (protocol no. 02/2549). Before the experiment, we kept mice undisturbed for 5 days to avoid the effect of stress from transportation.
Embryo collection
Five- to seven-week old ICR female mice were superovulated by an intraperitoneal injection (IP) of 5 IU pregnant mare serum gonadotropin (PMSG; Sigma Chemical, MO, USA), followed 48 h later by an IP injection of 5 IU human chorionic gonadotropins (HCG; NV Organon, The Netherlands). Superovulated females were mated with 10- to 12-week-old ICR males. They were checked for mating by the presence of vaginal plugs 16 h later. Forty h after HCG, 2-cell embryos were collected from the oviducts by flushing with phosphate-buffered saline solution (PBS; GIBCO, NY, USA). Embryos were pooled in small drops of cleavage medium (Cook, Australia) under oil (Medicult, Denmark) at 37°C in an atmosphere of 6% CO2, 5% O2 and 89% N2. They were randomly divided into 3 groups: non-frozen control (group 1), vitrification (group 2) and slow programmable freezing (group 3). Only 2-cell embryos with intact zona and nearly equal blastomeres without fragmentation were used in the experiments.
Closed-system solid surface vitrification
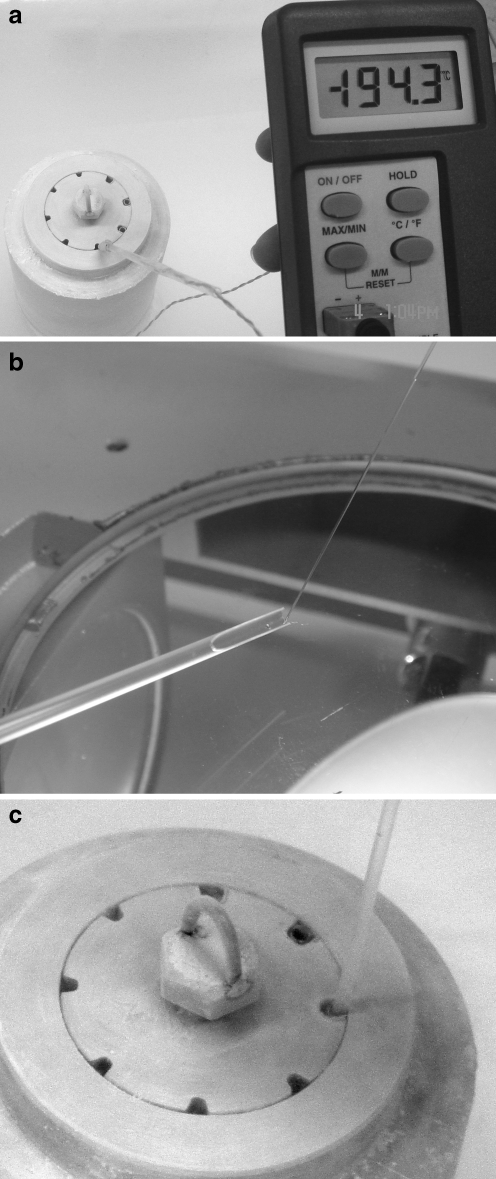
Our home-made vitrification system consisted of two aluminum cylinders. The inner one had eight grooves along its surface that could accommodate eight 0.5-mL straws in a vertical position. The inner cylinder was designed to fit into a cavity in the outer cylinder (Fig. 1a). The aluminum cylinders were immersed into liquid nitrogen for 20 min before use. A 0.5-mL straw (IMV, France) was sealed at the end that contained the factory plug. The straw was inserted into a hole inside the pre-cooled aluminum cylinders, with its sealed end at the bottom. The lower one-third of the 0.25-mL straw was cut open longitudinally to make a hemi-straw, and used as an embryo container. The hemi-straw was cut at the other end to shorten its length so that it could be inserted completely into the outer 0.5-mL straw.
Fig. 1.
Vitrification device (a) and the hemi-straw containing embryos (b). The hemi-straw was inserted into a 0.5 mL straw that was pre-cooled inside an aluminum cylinder bathed in liquid nitrogen (c)
Mouse 2-cell embryos were transferred into a freezing solution, containing 10% ethylene glycol (EG), 10% dimethylsulfoxide (DMSO) and 10% fetal bovine serum (FBS) in phosphate buffered saline (PBS). After 10 min, they were transferred into 17.5% EG, 17.5% DMSO, 0.25 M trehalose and 10% FBS in PBS for 30 s. A finely drawn Pasteur pipette was used to transfer a group of five embryos in a volume of 1 µl or less onto the end of the hemi-straw. The hemi-straw was immediately inserted into a pre-cooled 0.5 mL straw inside the aluminum cylinders (Fig. 1b and c). The other end of the 0.5-mL straw was sealed before storage in a liquid nitrogen (LN2) tank.
The embryos were thawed by cutting open the end of the 0.5-mL straw. A pair of fine forceps were used to grasp the inner hemi-straw and immerse it into a solution of 0.25 M trehalose with 20% FBS at 37°C. After each 3-min period, the embryos were transferred sequentially into 0.1, 0.05 and 0 M trehalose with 20% FBS at 37°C. Finally, the embryos were washed in cleavage medium (Cook, Australia) and observed for survival. A 2-cell embryo with at least one intact blastomere was defined as a surviving embryo.
Slow programmable freezing
Embryos were exposed to PBS supplemented with 20% FBS at 37°C and left to cool down slowly for 5 min to room temperature (25°C). They were then transferred into 1.5 M propanediol (PROH; Sigma Chemical, USA) in PBS supplemented with 10% FBS for 15 min at room temperature and then into the same solution with 0.1 M sucrose. No more than five embryos were loaded into each 0.25-mL plastic straw (IMV, France). The straws were sealed with plastic plugs and inserted into a programmable freezer (KRYO 10, series III, Planer, USA). A cooling rate of −2°C/min was used until the temperature dropped to −7°C. After soaking for 7 min, manual seeding was induced at the upper column of the straw. The temperature was held for another 7 min at −7°C, before slowly cooling down to −30°C at a rate of −0.3°C/min. The straw was then rapidly cooled down at a rate of −30°C/min to −150ºC. At this stage the straws were plunged into liquid nitrogen.
The embryos were thawed by removing the straw from the liquid nitrogen and immersing it into a 37ºC water bath until the ice melted. Both ends of the straw were cut open with a sterile pair of scissors. The content of the straw was emptied into a sterile Petri dish. The embryos were located and immediately transferred into 1 M PROH supplemented with 0.2 M sucrose and 10% FBS. The cryoprotectants were removed in a stepwise fashion by transferring the embryos into 0.5 M and 0 M PROH supplemented with 0.2 M sucrose and 10% FBS, and PBS supplemented with 10% FBS. Finally, the embryos were washed through cleavage medium (Cook, Australia) and observed for survival.
Mouse embryo culture
Surviving embryos were cultured in groups of 10 in 25 µl drops of cleavage medium under oil at 37ºC in a humidified atmosphere of 6% CO2, 5% O2 and 89% N2. After 24 h of culture, the embryos were observed for further cleavage and then transferred into blastocyst medium (Cook, Australia). After 72 h of culture, they were assessed for blastocyst and hatching blastocyst development.
Blastocyst staining
Fifteen expanded blastocysts from each group were randomly selected. They were exposed to 0.4% pronase in Earle’s balanced saline solution (EBSS; GIBCO BRL, NY, USA) to remove zona pellucida, and washed with PBS. The blastocysts were then stained with Bisbenzimide (Hoechst 33258 Fluorochrome, Sigma, USA) for 15 min at room temperature. Embryos were transferred into a drop of glycerol on a clean slide and covered with a coverslip. The number of nuclei in each blastocyst was counted under ultraviolet light using a fluorescence microscope (Nikon E600 epifluorescence microscope) equipped with the LUCIA FISH program (Laboratory Imaging, Czech) at a magnification of 400X. The counting was performed level by level, by changing the focus accordingly.
Intrauterine blastocyst transfer
Female recipient mice were treated with gonadotropins as previously described and were placed with vasectomized males. They were examined the next day for the presence of a vaginal plug. If present, they were designated as day 1 pseudopregnant mice. Blastocysts from control and frozen/thawed groups were transferred into the uteri of recipients on day 3 of pseudopregnancy. The method of transfer was as described by Hogan et al. [10]. Briefly, the recipients were anesthetized by IP injection of 3 mg Ketamine HCl (Calypsol; Gedeon Richter, Budapest, Hungary) and 0.3 mg Xylazine (Xylitol; Farvet, Bladel, Holland). A dorsal incision was made to pull the uterine horn out. A 25-gauge needle was used to make a hole in the uterus near the uterotubal junction. After removing the needle, a Flexipet denuding pipette (120 µm inner diameter), attached to a Flexipet Adjustable Handle Set (Cook, Brisbane, Australia), loaded with seven blastocysts, was inserted 5 mm into the uterine horn and the blastocysts were gently expelled with minimal amount of culture medium. The recipients were sacrificed on day 15 post-transfer to evaluate the viable fetuses and implantation marks.
Statistical analysis
Stata Statistical Software version 8.2 (Stata Corporation, College Station, Texas) was used for data analysis. Chi-square tests, complemented by Chi-square partition for the analysis of difference, were used to compare the proportions of survived embryos, those that underwent further cleavage, and those that developed into hatching and hatched blastocysts between the two freezing groups and controls. Embryos that were lost during the thawing process were reported and treated as non-surviving embryos in the final analyses. The average number of nuclei in the blastocysts in each group was compared by using analysis of variance (ANOVA). A two-tailed P < 0.05 was considered statistically significant.
Results
Immediate survival and further development
Three hundred and forty-eight 2-cell mouse embryos were randomly allocated as non-frozen control (116 embryos), vitrification (116 embryos) and slow programmable freezing (116 embryos) groups.
The immediate survival rate after freezing/thawing of embryos in the vitrification group (111/116; 95.7%) was significantly higher than that (90/116; 77.6%) in the slow freezing group (Chi-square test, p < 0.001). Partition of 3 × 2 Chi-square tests showed that further cleavage and blastocyst formation rates in the vitrification groups (105/116; 90.5% and 92/116; 79.3%, respectively) were significantly higher (p < 0.001) than those in the slow freezing group (82/116; 70.7%, and 68/116; 58.6%, respectively). However, further cleavage and blastocyst formation rates in the vitrification group were not significantly different from those in the non-frozen control (112/116; 96.5% and 104/116; 89.6%, respectively; p > 0.05).
Total number of cells in the resulting blastocysts
The average number of cells in the blastocysts, which derived from the frozen/thawed 2-cell embryos in the vitrification group, was not significantly different from that in the control (59.2 ± 8.0 and 55.7 ± 3.9, respectively), but it was statistically higher than that in the slow freezing group (46.4 ± 8.8, p < 0.005).
Pregnancy and implantation rates
Blastocysts from the control (n = 56), vitrification (n = 56) and slow-freezing (n = 56) groups were transferred into 12 pseudopregnant mice (4 mice per group). There was no difference (Fisher exact test, p = 0.99) in the pregnancy rate in the control (3 out of 4 recipients), vitrification (3 out of 4 recipients), and slow-freezing groups (2 out of 4 recipients). The implantation rate was not statistically different (P = 0.380) in the control (16/56 or 28.6%), vitrification (15/56 or 26.8%) and slow-freezing groups (10/56 or 17.9%). The number of viable fetuses was also not significantly different (P = 0.752) in the control (12/16 or 75%), vitrification (11/15 or 73%) and slow-freezing (6/10 or 60%) groups.
Cooling rate of vitrification
The temperature inside the straw was measured by a stainless steel temperature probe (MicroTherma 2, Rototherm, Dublin), every 5 s until stable. The temperature inside the straw dropped from 4°C down to −76.4°C, −94.8°C, −124.8°C, −153°C, −166.5°C, −174.3°C, −185°C, −190°C and stabilized at −194°C. The cooling rate was calculated to be approximately −964°C/min during the first 5 s, an average of −306.4°C/min during the next 15 s, an average of −128°C/min during the following 15 s, −60°C/min during the subsequent 5 s and finally −36°C/min during the last 5 s. The total time needed for the whole cooling process from 4°C down to −194°C was 45 s.
Cost comparison with commercial vitrification kits
The costs of 3 commercially available vitrification kits in Thailand are a) Cryotop Vitrification Kit (Kitazato BioPharma, Shizuoka, Japan) for 20,400 Bht (595.31 US dollars); b) SAGE Vitrification kit (CooperSurgical, Trumbull, CT) for 24,075 Bht (702.55 US dollars); and c) McGill Cryoleaf (Medicult, Jyllinge, Denmark) for 10,486 Bht (306 US dollars). The cost of our in-house vitrification and thawing media is 1,184 Bht (34.55 US dollars) and the cost of other consumables (including syringe filters, straws, tubes, etc) is 640 Bht (18.68 US dollars). Together, our in-house kit costs 53.23 US dollars.
Discussion
Many studies have been done to optimize the techniques for embryo cryopreservation. Most experiments depend on an animal model to avoid the legal, ethical and practical problems of dealing with scarce and precious human embryos. Mouse is the most commonly used animal model because the results can be broadly applied to humans [11, 12]. Moreover, mouse can provide a large number of embryos for experiments in a short period of time.
In this study, cleavage-stage embryos were chosen because we routinely cryopreserved the 4- to 8-cell stage human embryos in our clinical ART program. Mouse 2-cell stage was specifically selected because it corresponds best to human 4- to 8-cell stage, with regard to morphology and size of the blastomeres [13]. Moreover, the 2-cell stage can be considered as a sub-optimal developmental stage [14], and it is the stage more sensitive to cryodamage than any other cleavage stage [15]. Therefore, the detrimental effect of different cryopreservation methods should become more obvious.
Slow programmable freezing in our study gave a comparable survival and blastocyst development rate to other studies (77.6% versus 50%–87.6% and 58.6% versus 32.8%–76.4%, respectively) [13, 15, 16]. For our vitrification system, the survival (95.7%) and blastocyst development rates (79.3%) were higher than those reported in the conventional vitrification methods (60.3%–85% and 22%–38%) [13, 15, 17, 18]. Although the cooling rate achieved by our system (−964°C/min) was much lower than those of the container-less systems, such as the open-pulled straw (OPS; − 2,000°C/min), the survival and blastocyst development rates were comparable to those reported in the literature (87.5–96.3% and 62.2–68.9%, respectively) [18].
It was possible that at a given concentration of cryoprotectant, there was a limit beyond which an increase in the cooling rate would not result in a higher cryosurvival rate. Indeed, Nowshari and Brem [19] showed that increasing the freezing rate of mouse embryos from 1,200°C/min to 10,300°C/min was of no advantage to their vitrification system. Kuleshova and Shaw [20] reported that vitrification in a double straw, with a cooling rate of 400°C/min, was very effective for the cryopreservation of mouse embryos. It was also shown that a moderately low cooling rate of 120°C/min was effective for the vitrification of human embryos at all stages of development [11]. Kuleshova and Lopata [21] suggested that a cooling rate of − 400°C/min was appropriate for the vitrification of human immature oocytes and blastocysts.
In vitrification, the speed of cooling is inversely related to the concentration of cryoprotectants in the freezing medium. A higher cooling rate apparently allows a beneficial reduction in the concentration of the cryoprotectants [22]. However, a vitrification medium consisting of 15%–20% EG and 15%–20% DMSO, as used in this study, has been shown to possess very low toxicity in the oocytes and embryos of many species [23–26]. A further decrease in the concentration of this medium might not necessarily result in a better vitrification system.
There are several ways to assess the quality and viability of the frozen-thawed embryos. In this study, we used immediate survival rate, further cleavage rate post-thaw, blastocyst formation rate and total cell count in the blastocyst as our outcomes. The study showed that vitrification gave superior results to slow cooling in all of these aspects. Despite the fact that vitrified embryos were exposed to non-incubator conditions during the “handling” necessary for vitrification, they had cleavage rate, blastocyst rate and total cell count in the blastocyst comparable to that of the non-frozen control. The transfer of vitrified embryos into pseudopregnant mice confirmed that embryo viability was not different from non-frozen control.
One major disadvantage of the container-less systems is that embryos in the vitrification medium come into direct contact with liquid nitrogen, which can be a potential source of contamination. One needs to balance the use of a vitrification system that provides an extremely rapid cooling rate with a slower one that is less prone to contamination. As our embryos were isolated from the liquid nitrogen by a sealed straw, the potential risk of cross-contamination during cryopreservation could be greatly reduced or eliminated. In addition, our method was less costly, as all materials and instruments were made or modified in-house. In fact, it costs 6–13 times less than the commercially available vitrification kits.
Conclusions
Closed system solid surface vitrification was more effective than conventional slow freezing in the cryopreservation of mouse 2-cell embryos. Further study should be done before this method can be applied for clinical use in human embryo cryopreservation.
Acknowledgements
This study was supported by a grant from the Faculty of Medicine Endowment Fund for Medical Research, Chiang Mai University, Thailand.
Footnotes
Capsule
Closed system solid surface vitrification was more effective than conventional slow freezing in the cryopreservation of mouse 2-cell embryos.
References
- 1.Anderson RA. Policy and Practice sub-Group of the British Fertility Society. A strategy for future reproductive services for survivors of cancer. Hum Fertil (Camb). 2003;6:113–5. [DOI] [PubMed]
- 2.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65:236–44. [DOI] [PubMed]
- 3.Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their development competence. Mol Reprod Dev. 1996;45:503–12. [DOI] [PubMed]
- 4.Lane M, Bavister BD, Lyons EA, Forest KT. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol. 1999;17:1234–6. [DOI] [PubMed]
- 5.Vajta G, Booth PJ, Holm P, Greve T, Callesen H. Successful vitrification of early stage bovine in vitro produced embryos with the open pulled straw (OPS) method. Cryo-Letters. 1997;18:191–5.
- 6.Liebermann J, Tucker M, Graham J, Han T, Davis A, Levy MJ. Blastocyst developement after vitrification of multipronucleate zygote using the flexipet denuding pipette (FDP). Reprod Biomed Online. 2002;4:148–52. [DOI] [PubMed]
- 7.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. [DOI] [PubMed]
- 8.Angle MJ. Survival and re-expansion of mouse blastocyst following vitrification in two FDA-approved closed devices with and without assisted shrinkage. Fertil Steril. 2007;88(Suppl 1):S90–1. [DOI]
- 9.Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46:146–52. [DOI] [PubMed]
- 10.Hogan B, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Laboratory; 1986. p. 142–5.
- 11.Mukaida T, Wada M, Takahashi K, Pedro PB, An TZ, Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13:2874–9. [DOI] [PubMed]
- 12.Quinn P, Kerin JF. Experience with the cryopreservation of human embryos using the mouse as a model to establish successful techniques. J In Vitro Fert Embryo Transf. 1986;3:40–5. [DOI] [PubMed]
- 13.Hredzak R, Ostro A, Maracek I, Kacmarik J, Zdilova V, Vesela J. Influence of slow-rate freezing and vitrification on mouse embryos. Acta Veterinaria Brno. 2005;74:23–7.
- 14.Ohashi A, Minami N, Imai H. Nuclear accumulation of cyclin B1 in mouse two-cell embryos is controlled by the activation of cdc2. Biol Reprod. 2001;65:1195–200. [DOI] [PubMed]
- 15.Uechi H, Tsutsumi O, Morita Y, Takai Y, Taketani Y. Comparison of the effects of controlled-rate cryopreservation and vitrification on 2-cell mouse embryos and their subsequent development. Hum Reprod. 1999;14:2827–32. [DOI] [PubMed]
- 16.Lee YI, Kwon YS, Park HJ. The experimental study on cryopreservation of mouse embryo. Korean J Fertil Steril. 2001;28:55–64.
- 17.Valojerdi MR, Movahedin M, Hosseini A. Improvement of development of vitrified two-cell mouse embryos by vero cell coculture. Assist Reprod Genet. 2002;19:31–8. [DOI] [PMC free article] [PubMed]
- 18.Yuan YH, HongMing T, Chong Z, QiongHua H. Study on cryopreservation of 2-cell mouse embryos by straw or OPS vitrification. Chinese J Animal Science. 2004;40:3–6.
- 19.Nowshari MA, Brem G. Effect of freezing rate and exposure time to cryoprotectant on the development of mouse pronuclear stage embryos. Hum Reprod. 2001;16:2368–73. [DOI] [PubMed]
- 20.Kuleshova LL, Shaw JM. A strategy for rapid cooling of embryos within a double straw to eliminate the risk of contamination during cryopreservation and storage. Hum Reprod. 2000;15:2604–9. [DOI] [PubMed]
- 21.Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril. 2002;78:449–54. [DOI] [PubMed]
- 22.Bielanski A. Non-transmission of bacterial and viral microbes to embryos and semen stored in the vapour phase of liquid nitrogen in dry shippers. Cryobiology. 2005;50:206–10. [DOI] [PubMed]
- 23.Begin I, Bhatia B, Baldassarre H, Dinnyes A, Keefer CL. Cryopreservation of goat oocytes and in vivo derived 2- to 4-cell embryos using the cryoloop (CLV) and solid-surface vitrification (SSV) methods. Theriogenology. 2003;59:1839–50. [DOI] [PubMed]
- 24.Sheehan CB, Lane M, Gardner DK. The CryoLoop facilitates re-vitrification of embryos at four successive stages of development without impairing embryo growth. Hum Reprod. 2006;21:2978–84. [DOI] [PubMed]
- 25.Yang QE, Hou YP, Zhou GB, Yang ZQ, Zhu SE. Stepwise in-straw dilution and direct transfer using open pulled straws (OPS) in the mouse: a potential modle for field manipulation of vitrified embryos. J Reprod Dev. 2007;53:211–8. [DOI] [PubMed]
- 26.Cremades N, Sousa M, Silva J, Viana P, Sousa S, Oliveira C, et al. Experimental vitrification of human compacted morulae and early blastocysts using fine diameter plastic micropipettes. Hum Reprod. 2004;19:300–5. [DOI] [PubMed]