Abstract
Purpose
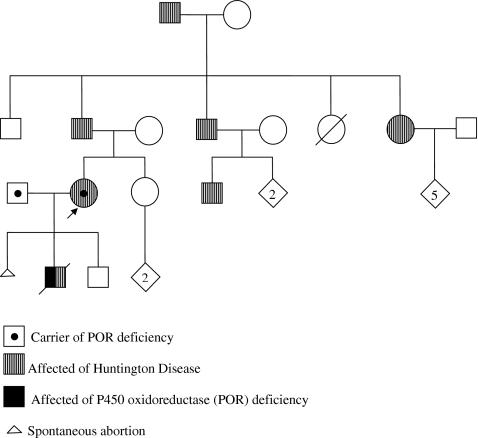
Description of the confluence of different molecular techniques to detect three different mutations in one cell. The man carries a 20 base pair insertion in exon 12 of the POR gene (c.1551_1552ins20), and the woman carries a point mutation in exon 8 of the POR gene (c.859G>C) plus a triplet repeat expansion in the HTT gene.
Methods
Huntington Disease (HD) had to be diagnosed using short tandem repeat (STR) markers linked to the HTT gene. The mutation c.1551_1552ins20 was analyzed by fragment size and c.859G>C was minisequenced. Furthermore, STR markers linked to the POR gene were included to support the diagnosis of P450 oxidoreductase (POR) deficiency.
Results
Nine embryos were diagnosed in total: three as POR deficiency affected, two as HD affected, one as POR deficiency and HD affected, and two as carriers of the paternal POR deficiency mutation and healthy for HD. These two last embryos were transferred but no pregnancy was achieved.
Conclusions
A successful procedure combining direct and indirect methods for the detection of three different mutations in a single cell has been achieved for the first time.
Keywords: Preimplantation genetic diagnosis, Monogenic disease, P450 oxidoreductase deficiency, Huntington Disease, Molecular PGD
Introduction
Preimplantation Genetic Diagnosis (PGD) using molecular techniques has been used successfully since 1990 to avoid the transmission of single gene disorders to offspring [1]. Since then, the number of world-wide uses of molecular PGD has shown a tremendous increase [2]. The development of different technologies allows accurate diagnosis and the extension of the application of PGD, not only to the most common single gene disorders but also to more complicated cases such HLA typing. In fact, HLA typing has been combined with PGD for monogenic blood diseases or aneuploidy screening [3–5]. Also, some cases involving use of PGD for monogenic disorders plus age-related aneuploidies screening have been reported [6].
The increase in PGD case demand has encouraged the development of standardized molecular PGD protocols for the more common diseases by using a set of linked short tandem repeat (STR) microsatellite markers in order to avoid the need for a specific set up for each couple [7, 8]. The only requirement that couples must meet is to have an informative family for the specific disease STR marker set. The problem with this standardisation is that many couples do not meet the requirements because they may have a very rare disorder not included in the most frequently dealt with diseases, they may not have an informative family or they may carry more than one genetic disease. In such cases, these families cannot benefit from these standardised techniques and specific direct methods are needed. The setting up is time-consuming and specific difficulties must be overcome, but this is the only option for PGD in these couples.
In this context, we present the application of PGD for two single gene disorders simultaneously in one couple. In this case, both parties are carriers of two different mutations in the POR gene, causing P450 oxidoreductase (POR) deficiency in compound heterozygosis. The woman is also a carrier of a pathological expansion of the triplet that causes Huntington Disease in adult age.
The P450 oxidoreductase (POR) deficiency (OMIM #201750) is an extremely rare autosomal recessive disease caused by a disorder of steroidogenesis. Approximately 40 cases have been reported since the first mutations were described in 2004 [9]. The characteristic traits are steroid abnormalities, craniofacial, skeletal and urogenital anomalies and, commonly, a reduction of cognitive functions and delay in development (reviewed in [10]). This disorder often results in infant death. Genetically, POR deficiency is caused by mutations in the POR gene (7q11.2) and a panel of different mutations has been previously described [11].
Huntington Disease (HD: OMIM +143100) is a late-onset autosomal dominant disease that causes progressive disturbances of motor (typical chorea), cognitive, and psychiatric abilities. The mean age of onset is between 35 to 44 years and the average survival time is 15 to 18 years after onset [12]. The prevalence of HD is 15 cases per 100,000 people in some populations, mostly of western European origin [13]. From the genetic point of view, HTT (alias HD or IT15) is the only gene associated with HD and a CAG trinucleotide repeat expansion in exon 1 is the only mutation that has been observed since it was characterised in 1993 [14]. Alleles in the HTT gene are classified as normal, premutation, or pathologic depending on the number of CAG repeats: i) normal alleles: 26 or less CAG repeats, ii) premutation alleles: 27–35 CAG repeats, and iii) pathologic alleles: 36 or more CAG repeats. People who have a pathologic allele are considered at risk of developing HD in their lifetime.
In this couple, the probability of getting a healthy or asymptomatic carrier embryo is 37.5%, representing PGD as a valid option. Simultaneous detection of two genetic diseases and the three different disease-causing mutations present in the couple offers them an alternative to prenatal diagnosis and termination of pregnancy.
The aim of this paper is to show for the first time, the development of a robust diagnostic scheme for the simultaneous detection of two genetic diseases caused by three different mutations by combining three different techniques (two direct and one indirect) in single blastomeres from two different PGD cycles.
Materials and methods
Family history
The candidate couple comprises a 36 year old woman and a 38 year old man, both with normal phenotypes. They were subjected to comprehensive genetic counselling including a review of their family histories, compilation of clinical and genetic reports, explanation of genetic disorders and evaluation of PGD request and IVF cycle in combination with biopsy procedures. The genetic risk, the success rates, the risk of misdiagnosis and the importance of prenatal diagnosis in case of pregnancy were specifically discussed. Finally, our Clinical Geneticist discussed the complete process with the patients and answered their queries. The couple signed an informed consent form for PGD testing.
The genetic history (Fig. 1) revealed that the woman carries a pathological expansion of 45 repeats of the CAG triplet in exon 1 of the HTT gene. This results in the production of a protein with an abnormally expanded polyglutamine tract. At the time of consultation she was asymptomatic. In addition to the triplet repeat expansion in HTT she carries the mutation c.859G>C (A287P) in exon 8 of the POR gene, representing a guanine to cytosine change in nucleotide position 859 of the cDNA. This change results in an alanine to proline substitution in position 287 of the protein P450 oxidoreductase (P450) sequence and a reduction of its function. Moreover, the man carries the mutation c.1551-1552ins20 representing a 20 base pair insertion in exon 12 of the POR gene. This causes reading frame disruption that grossly disrupts the flavin mononucleotide and NADPH-binding sites of P450 oxidoreductase, inducing premature stop codon upstream of residue 616 [11].
Fig. 1.
Familial genetic history. The arrow indicates the consultant patient
The reproductive history included three pregnancies, the first one of which resulted in an early non-diagnosed spontaneous abortion and the two others being an affected and normal son respectively. The affected son was clinically and genetically diagnosed with POR deficiency. He was a compound heterozygote (c.859G>C/c.1551-1552ins20) and died when he was 6 years old. The normal son was diagnosed as genetically normal for HD (prenatal diagnosis) and clinically asymptomatic for POR deficiency. Later, the informativity study previous to PGD confirmed the genetic status (normal/normal) for the POR gene (see “Results”).
Preclinical studies
Blood samples in EDTA were requested from parents and son. DNA extraction was carried out following the protocol described in the High pure PCR template preparation kit (Roche, Switzerland).
Preclinical work-up began with the informativity study, confirming the genetic status of the couple. Oligonucleotide primers for exon 8 and exon 12 of POR gene were designed to amplify the regions that contained the mutations c.859G>C and 1551-1552ins20 respectively. Microsatellite markers were chosen according to their heterozygosity and distance to the POR gene. PCR amplification of exon 8 and 12 of gene POR was performed using the outer and inner oligonucleotide primers listed in Table 1. Two STR markers closely linked to the POR deficiency gene were tested and the informative one was included to avoid possible misdiagnosis resulting from allele drop out (ADO). ADO is a well-defined cause of false homozygous diagnosis induced by the lack of amplification of one allele in single cell PCR [15–17].
Table 1.
Primer sequences used for preimplantation genetic diagnosis of A) P450 oxidoreductase (POR) deficiency and B) Huntington Disease
| A) | POR-E8 (V287P) | POR-E12 (1551_1552ins20) |
| Fout | 5′GAGACTCAGATCAAAGCCCC3′ | 5′GCCCTCCCCACAGGTCCAC3′ |
| R | 5′CAGGTGCATGAGGTGGCGCTC3′ | 5′CATGTAGACAGGCGTGGTGGC3′ |
| Fin | 5′ PET-GCCGCTCACTGTGCTTCTCTCC3′ | 5′ 6-FAM-GGTTGTGGAGTACGAGACCAAGGC3′ |
| Miniseq | 5′GCCCCTTTGATGCCAAGAATCCGTTCCTG3′ | N/A |
| B) | Exon 1 HD triplet (HD2) | |
| Fout | 5′CCATTCATTGCCCCGGTGCTGAC3′ | |
| R | 5′GGCGGCTGAGGAAGCTGAGGA3′ | |
| Fin | 5′-6-FAM-ATGGCGACCCTGGAAAAGCTGATGAA3′ |
Fout forward outside primer; Fin forward inside primer; R reverse primer; Miniseq minisequencing primer
The couple were non-informative for HTT repeat number in exon 1 because the healthy allele of the woman had the same repeat number as one of the two alleles of her husband. This fact made it impossible for direct diagnosis, as the healthy maternal allele in the embryos would not be distinguishable from the paternal one. As sometimes the expanded allele cannot be amplified due to its size, therefore only one allele is detectable and a healthy homozygote where maternal and paternal alleles are the same size cannot be distinguished from an HD embryo. In order to avoid this problem, different STR markers linked to the HTT gene were tested and two of them were chosen to determine the haplotypes linked to Huntington Disease (Table 2). Oligonucleotides were tested in order to discard possible human DNA contamination.
Table 2.
Primer sequences for the short tandem repeats (STR) markers used in preimplantation genetic diagnosis of P450 oxidoreductase (POR) deficiency and Huntington Disease
| POR deficiency | |||
| Name | Heterozygosity (%) | POR gene distance (Mb) | |
| D7S2518 | 0.77 | 0.16 | Fout: |
| 5′ACTGACTTGTAGGGATGTCCATG3′ | |||
| R: | |||
| 5′GGATTATATCCTTCCATGCCTTGTG3′ | |||
| Fin: | |||
| 5′NED-GCAGGAAGTTGCAGAGTAGAGTG3′ | |||
| Huntington Disease | |||
| Name | Heterozygosity (%) | HD gene distance (Mb) | |
| D4S127 | 0.71 | 0.03 | Fout: |
| 5′CGTGCAGTCCACCCTGCCCTG3′ | |||
| R: | |||
| 5′GTCCCTTGCATGCCCTGGCT3′ | |||
| Fin: | |||
| 5′ NED-GACCTCTGTTTGCAATCCATTTTGC3′ | |||
| D4S412 | 0.77 | 0.13 | Fout: 5′CCTGTGTCCAACATCTCTCC3′ |
| R: | |||
| 5′GCTAAGATATGAAAACCTAAGGGA3′ | |||
| Fin: | |||
| 5′ VIC-CACTACCGCCAGGCACTGG3′ | |||
Fout forward outside primer; Fin forward inside primer; R reverse primer
Lymphocyte validation and blastomere analysis
The reliability of these protocols was evaluated before clinical application following international guidelines [2]. The protocol was performed first on genomic DNA, then on 1 ng/µL DNA and finally on single lymphocytes collected from both parents.
Lymphocytes were isolated using Histopaque-1007 (Sigma-Aldrich, Germany) according to the manufacturer’s protocol. Single lymphocytes were isolated under a stereo microscope in a laminar flow cabinet. Only one lymphocyte, from the final stock solution (see Histopaque-1007 protocol), was aspirated with an extremely fine Pasteur pipette by a mouth-controlled system. The single cell was rinsed in three micro-drops (10 µl) of Biopsy Medium (Medicult, Denmark). Single lymphocytes were placed into 0.2 ml PCR tubes containing 2.5 µl of alkaline lysis buffer (200 mM NaOH, 50 mM DTT) and frozen immediately at −80°C until required for PCR. An aliquot of the final micro-drop wash was used as a negative control. A small quantity of stock solution of lymphocytes was used as a positive control.
A first round of multiplex PCR was performed containing the outer forward and reverse primers for the amplification of the gene regions involved in mutations and linked STR markers (POR-E8, POR-E12 and D7S2518 for POR deficiency and D4S127 and D4S412 for Huntington Disease). The first round of multiplex PCR was followed by a separate second round heminested PCR for each fragment with forward inner and reverse primers, where the forward inner one is fluorescently labelled. Controls to detect possible PCR contamination were included: a negative control for each round of PCR and positive controls containing DNA from the PCR technician and, lymphocyte isolation or biopsy technician, depending on which stage of the PGD had been reached.
The molecular analysis protocol was the same for lymphocytes (preclinical set up) and blastomeres (PGD). Single cells in alkaline lysis buffer were thawed at RT and heated at 65°C for 10 min. Lysed cells were neutralised with 2.5 µL of neutralising buffer (500 mM Tricine pH 4.9) and subjected to multiplex PCR analysis. First round PCR was carried out in a total volume of 25 µL containing 200 µM dNTP, 1.5 mM MgCl2, 1X PCR buffer, 1 unit AmpliTaq Gold polymerase (Applied Biosystems, U.S.A) and 0.4 µM each of the outer forward and reverse primers. Following first round PCR with outer primers, 1 µL was used as template for separate second round PCR reactions with inner forward and reverse primers in a total volume of 10 µL. PCR reactions were undertaken using a 9700 PCR system (Applied Biosystems, U.S.A.). Twenty-five thermal cycles were performed for the outer primers and 50 for the inner primers at 95°C for 1 min, 50°C for 45 s, and 72°C for 1 min. The cycling was preceded by a 5 min initial denaturation step at 95°C and ended with an additional elongation step of 7 min at 72°C.
Five microlitres of amplified POR-E8 inner products were purified with 2 µL of ExoSap-IT. Minisequencing reactions were set up using the SnapShot™ multiplex kit (Applied Biosystems, U.S.A), following manufacturer’s instructions. Resulting products were treated with calph intestinal alkaline phosphatase in order to remove unincorporated ddNTPs, incubating at 37°C for 1 h and heating to 75°C for 15 min to inactivate the enzyme.
Amplified inner products were diluted 1:10 and 1 µL was added to 9 µL of Hi-Di formamide (Applied Biosystems, U.S.A) and 0.25 µL of GeneScan™ 500-Liz (Applied Biosystems, U.S.A) for fragment analysis or GeneScan™ 120-Liz for minisequencing. After denaturation for 5 min at 95°C and fast cooling on ice, products were capillary electrophoresed in an automated genetic analyser 3730xl (Applied Biosystems, U.S.A). Results were analysed with Genemapper software (Applied Biosystems, U.S.A).
In-vitro fertilization (IVF) and embryo biopsy
Patients were referred to a concerted assisted reproduction clinic in order to perform the IVF cycle, embryo biopsy and embryo transfer procedures.
The woman underwent a routine superovulation procedure. Controlled ovarian hyperstimulation was performed using the long GnRH agonist protocol. The patient was stimulated with recombinant human follicle stimulating hormone (FSH) (Gonal F; Serono, Germany) after pituitary function was down-regulated with gonadotropin releasing hormone (GnRH-agonist), starting on the 21st day of the cycle preceding the IVF treatment. Follicular development was monitored using serial vaginal ultrasound and serum oestradiol levels. Ultrasound-guided transvaginal oocyte retrieval was performed 34–36 h after hCG administration.
Cumulus and corona radiata cells were completely removed by enzymatic and mechanical procedures. Subsequently, ICSI was performed and assessed for normal fertilization after 17 h. Embryos were cultured in vitro until day +3 post-insemination.
On day +3, the zona pellucida dissection was performed to create a hole by mechanical techniques using special micropipettes (PZD Micropipette, Humagen, VA, USA). The biopsy procedure was performed in embryos at the 6–8 cell stage using a 35 mm ID micropipette removal (MBB-BP-SM-30 Micropipette, Humagen). Two blastomeres from each embryo were removed if the embryo had more than six cells. Only one cell was biopsied in embryos containing less than six cells. Each blastomere with a clearly visible nucleus was washed in 5 µl droplets of Ca2+- and Mg2+- free medium under mineral oil supplemented with 5 mg/ml of BSA solution and then transferred to a 200 µl PCR tube with 2.5 µl alkaline lysis buffer (ALB) and frozen at −80°C. A small volume of biopsy medium was used as a negative control. A negative control was used per blastomere. The frozen PCR tubes were sent by express courier to the genetics laboratory.
Results
Set up and informativity test
Preliminary genetic analyses on genomic DNA from the couple, healthy son and maternal grandfather were carried out in order to verify the genetic status of each family member and to determine the STR alleles linked to POR deficiency and HD mutations (Fig. 2). As expected, the mutations in the POR gene were confirmed in both members of the couple and were absent in the healthy son. This information was useful to assign the STR marker D7S2518 alleles linked to each mutation.
Fig. 2.
Simultaneous preimplantation genetic diagnosis (PGD) for P450 oxidoreductase (POR) deficiency and Huntington Disease (HD). a Pedigree showing data from the family members involved in the informativity study and embryos from the first PGD cycle. The fine lines represent the normal allele for both POR deficiency and HD. The dotted lines represent the paternal c.1551_1552ins20-bearing haplotype for POR deficiency. The discontinuous lines represent the maternal c.859G>C-bearing haplotype for POR deficiency. Haplotypes for the STR markers are underneath the symbol of each family member or embryos. Numbers represent STR marker allele length in base pairs. The minisequenced nucleotide position in the POR gene is indicated by G (Guanine) or C (Cytosine), corresponding to normal or mutated nucleotide respectively. b Data from the second PGD cycle. EXP: expansion; ET: embryo transfer; ADO: allelic drop out, na: not analysed
As the healthy HD allele of the mother was the same size as one of the father alleles, a direct analysis of HD was not possible. Hence, the D4S412 and D4S127 alleles of the maternal grandfather (HD affected) that matched the mother’s ones were assigned as linked to HD and used for indirect diagnosis of HD of the couple’s embryos.
Once the strategy of diagnosis to detect the three mutations in the couple was set up, validation of the techniques was carried out with 20 single isolated lymphocytes from each parent. Amplification failure and ADO rates for each mutation containing amplified fragments or STR markers were estimated. PCR efficiency for each STR marker was 50% for D7S2518, 75% for D4S127 and D4S412, 83% for the primer amplifying exon 8 of the POR gene and 100% for the primer amplifying exon 12 of the POR gene. ADO rates were 0% for exon 8, exon 12, D7S2518 and D4S127 and 15% for D4S412.
Clinical PGD cycles
Table 3 shows the IVF results of the two PGD cycles performed. In the first cycle, eleven oocyte-corona-cumulus complexes were retrieved. All of them were metaphase II stage oocytes and underwent ICSI. Nine oocytes fertilized and six reached the 6–8 cells stage on day +3. Two blastomeres were biopsied in five of the six embryos. Only one cell was biopsied from the sixth embryo. A total of eleven blastomeres were analysed. From the genetic point of view, results of the simultaneous PGD for POR deficiency and Huntington Disease are summarized in Fig. 2 and some examples are shown in Fig. 3. In the first cycle, embryo number 1 carried the maternal POR mutation and was HD affected, embryos number 2 and 5 carried the paternal POR mutation and were healthy for HD and embryos number 8 and 9 were carriers of both maternal and paternal POR mutations. The embryo number 11 failed to amplify and could not be diagnosed. Embryos number 2 and 5 (POR mutation carriers) were transferred but, unfortunately, no pregnancy was achieved.
Table 3.
Summary for the two preimplantation genetic diagnosis cycles
| Cycle 1 | Cycle 2 | |
|---|---|---|
| No. of OCC complexes | 11 | 7 |
| No. of MII oocytes | 11 | 6 |
| No. of ICSI | 11 | 6 |
| No. of 2PN zygotes | 9 | 6 |
| No. of biopsied embryos | 6 | 3 |
| No. of blastomeres analysed | 11 | 4 |
| No. of diagnosed embryos | 5 | 3 |
| No. of affected embryos (PORor HD) | 3 | 3 |
| No. of healthy embryos (POR or HD) | 0 | 0 |
| No. of carriers embryos (POR) | 2 | 0 |
| No. of embryos transferred | 2 | 0 |
| Results | No pregnancy | No transfer |
OCC oocyte-corona-cumulus, MII metaphase II oocytes, ICSI intracytoplasmic sperm injection, 2PN two pronuclei, POR P450 oxidoreductase (POR) deficiency, HD Huntington Disease
Fig. 3.
PGD results for some of the couple’s embryos. a Analysis of POR mutations and linked D7S2518 STR marker alleles. In exon 8, c.859 position was minisequenced: G represents Guanine and C represents Cytosine. In exon 12 the normal allele was noted as N and the mutated allele was noted as c.1551_1552ins20. Maternal D7S2518 allele linked to c.859G>G POR mutation is shown with a continuous line arrow and paternal allele linked to c.1551_1552ins20 POR mutation is shown with a dashed line arrow. b Analysis of D4S412 and D4S127 STR marker alleles closely linked to the HD gene. Maternal alleles linked to HD are shown with dotted line arrows
In the second PGD cycle, seven oocyte-corona-cumulus complexes were retrieved. Six of them were metaphase II stage oocytes and underwent ICSI. All oocytes were fertilized and only three reached the 6–8 cells stage on day +3. Two blastomeres were biopsied from one embryo. Only one cell was biopsied from the other two. Four blastomeres were analysed in total. One embryo was affected by POR deficiency (carrier of both maternal and paternal POR mutations), another embryo was a carrier of HD and the last embryo was affected of POR deficiency (compound heterozygote of the maternal and paternal POR mutations) and also HD carrier. No transfer was possible in this cycle.
Discussion
Preimplantation genetic diagnosis for couples at risk of pregnancies with serious genetic disorders is firmly established as a valid reproductive option for couples after appropriate genetic counselling. The list of couples demanding PGD increases exponentially and the published list of diseases for which a protocol has been developed is longer each year as shown by the number of annual publications [18]. The development of direct methods for specific mutations and diseases is expensive and time-consuming, which is why Molecular-PGD laboratories tend to standardize protocols for the more frequent rare diseases using a set of gene linked STR markers [8, 19, 20]. The only condition required for this approach is that couples must have an informative family for this set of STR markers in order to establish the haplotype linked to the familial disease. However, this request cannot always be fulfilled, for example in cases where couples have small families or only few affected members, in cases of de novo mutations, recessive autosomal diseases or in very infrequent rare diseases.
This case report is an example of ad hoc setting up. It was necessary to combine the application of direct and indirect diagnosis genetic methods to allow the detection of HD and POR deficiency mutations simultaneously in the same one embryonic cell. Huntington Disease is a relatively frequent rare disease whose detection is offered by many PGD centres worldwide but POR deficiency is an extremely rare recessive disorder with few affected families reported in the world. No PGD cycles for POR deficiency have been previously described.
The coexistence of two rare diseases in the same couple is extremely uncommon, although one case of simultaneous PGD for two single gene disorders has been previously reported [21]. In this case report, an indirect approach based on STR markers analysis was described. Even cases of PGD with a double factor, aneuploidy screening and single gene disorder, have been published [6, 22]. The authors performed the chromosome screening on the first polar body of the oocyte plus the monogenic mutation analysis on re-biopsied day +3 cleavage-stage embryos. Furthermore, single mutation detection in combination with one STR marker for chromosome 21 analysis as a Down’s syndrome screening has been reported [23]. However, the novelty of this work is found in the simultaneous detection of both conditions (meaning three different mutations) in the same one cell. We have used a direct diagnosis approach to detect a point mutation (c.859G>C) and a 20 base pair insertion (1551-1552ins20) for POR deficiency by minisequencing and fragment analysis respectively, plus haplotyping to detect HD.
Minisequencing to detect point mutations or fragment analysis for deletion/insertion detection is our first-choice procedure. It has proven to be accurate enough to detect single nucleotide substitutions in the context of PGD [24, 25]. This technique allows us to interrogate the exact position of a nucleotide change, genotyping the embryo. Furthermore, the direct diagnosis is supported by intragenic or flanking informative STR markers. When linked markers are used, extragenic distance to the gene has to be very short in order to detect eventual recombination events, reducing the risk of misdiagnosis. Moreover, the use of STR markers allows a diagnosis to be made in the case of ADO of the mutant allele or eventual minisequencing failure. In this particular case, minisequencing presents two main advantages. On one side, permits a combined strategy, analyzing the exact position of a point mutation and using concomitantly fragment analysis and indirect STR haplotype assays in order to detect the two other mutations. On the other hand, the use of minisequencing makes possible the use of less STR markers, reducing the complexity of the multiplex PCR reaction. The combination of multiple STR markers may affect adversely the amplification of a particular locus. In our experience we found incompatible mixtures of primers, making the diagnostic process complicated.
The development of specific procedures for each case is of great importance. In this sense, it is compulsory to achieve the maximum level of amplification efficiency, sensitivity, reliability and reproducibility during the preclinical work-up. Extremely rigorous laboratory procedures are mandatory in order to control the risks associated to single cell PCR as contamination, preferential amplification and ADO [16, 17].
In conclusion, the combination of different strategies based on PCR allows the simultaneous diagnosis of two diseases caused by a total of three different mutations in a single embryonic cell. From a genetic point of view, monogenic disease-causing mutations are very well established i.e., point mutations, small deletions or insertions, triplet expansions or large deletions. In this sense, the PGD for each single gene disorder can be based on robust techniques of mutation detection in single cells depending on the mutation nature, not on the disease itself. In this way, it is possible to develop PGD for any single gene disorder if the disease-causing mutation(s) is (are) genetically identified in the couple. The experience provided by this case allows us to be confident that PGD is universally applicable if different molecular techniques are combined.
Footnotes
A description of two PGD cycles for the simultaneous detection of two monogenic diseases in one couple: P450 oxidoreductase deficiency and Huntington Disease.
References
- 1.Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344:768–70. [DOI] [PubMed]
- 2.The Preimplantation Genetic Diagnosis International Society PGDIS. Guidelines for good practice in PGD: programme requirements and laboratory quality assurance. RBM Online. 2008;16:134–47. [DOI] [PubMed]
- 3.Fiorentino F, Biricik A, Karadayi H, Berkil H, Karlikaya G, Sertyel S, et al. Development and clinical application of a strategy for preimplantation genetic diagnosis of single gene disorders combined with HLA matching. Mol Hum Reprod. 2004;10:445–60. [DOI] [PubMed]
- 4.Verlinsky Y, Rechitsky S, Sharapova T, Laziuk K, Barsky I, Verlinsky O, et al. Preimplantation HLA typing with aneuploidy testing. RBM Online. 2005;12:89–100. [DOI] [PubMed]
- 5.Verlinsky Y, Rechitsky S, Laziuk K, Librach C, Genovese R, Kuliev A. Preimplantation genetic diagnosis for Pelizaeus-Merzbacher disease with testing for age-related aneuploides. RBM Online. 2006;12:83–8. [DOI] [PubMed]
- 6.Obradors A, Fernández E, Rius M, Oliver-Bonet M, Martínez-Fresno M, Benet J, et al. Birth of a healthy boy after a double factor PGD in a couple carrying a genetic disease and at risk for aneuploidy: case report. Hum Reprod. 2008;23:1949–56. [DOI] [PubMed]
- 7.Dreesen JC, Jacobs LJ, Bras M, Herbergs J, Dumoulin JC, Geraedts JP, et al. Multiplex PCR of polymorphic markers flanking the CFTR gene; a general approach for preimplantation genetic diagnosis of cystic fibrosis. Mol Human Reprod. 2000;6:391–6. [DOI] [PubMed]
- 8.Spits C, De Rycke M, Verpoest W, Lissens W, Van Steirteghem A, Liebaers I, et al. Preimplantation genetic diagnosis for Marfan syndrome. Fertil Steril. 2006;86:310–20. [DOI] [PubMed]
- 9.Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–30. [DOI] [PubMed]
- 10.Scott RR, Miller WL. Genetic and clinical features of P450 oxidoreductase deficiency. Horm Res. 2008;69:266–75. [DOI] [PubMed]
- 11.Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, et al. Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–49. [DOI] [PMC free article] [PubMed]
- 12.Harper B. Huntington disease. J Roy Soc Med Online. 2005;98:550. [DOI] [PMC free article] [PubMed]
- 13.Harper PS, Jones L. Huntington’s disease: genetic and molecular studies. In: Bates G, Harper P, Jones L, editors. New York, Oxford University Press; 2002. pp. 113–158.
- 14.Huntington’s disease collaborative research group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. [DOI] [PubMed]
- 15.Wilton L, Thornhill A, Traeger-Synodinos J, Sermon KD, Harper JC. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;1:1–8. [DOI] [PubMed]
- 16.Lewis CM, Pinêl T, Whittaker JC, Handyside AH. Controlling misdiagnosis errors in preimplantation genetic diagnosis: a comprehensive model encompassing extrinsic and intrinsic sources of error. Hum Reprod. 2001;16:43–50. [DOI] [PubMed]
- 17.Piyamongkol W, Bermúdez MG, Harper JC, Wells D. Detailed investigation of factors influencing amplification efficiency and allele drop-out in single cell PCR: implications for preimplantation genetic diagnosis. Mol Hum Reprod. 2003;9:411–20. [DOI] [PubMed]
- 18.Goossens V, Harton G, Moutou C, Scriven PN, Traeger-Synodinos J, Sermon K, et al. European Society Of Human Reproduction And Embryology PGD Consortium: ESHRE PGD consortium data collection VIII: cycles from January to December 2005 with pregnancy follow-up to October 2006. Hum Reprod. 2008;23:2629–45. [DOI] [PubMed]
- 19.Lledó B, Ten J, Rodriguez-Arnedo D, Llácer J, Bernabeu R. Preimplantation genetic diagnosis of X-linked retinoschisis. RBM Online. 2008;16:886–92. [DOI] [PubMed]
- 20.Gigarel N, Frydman N, Burlet P, Kerbrat V, Tachdjian G, Fanchin R, et al. Preimplantation genetic diagnosis for autosomal recessive polycystic kidney disease. RBM Online. 2008;16:152–8. [DOI] [PubMed]
- 21.Altarescu G, Brooks B, Margalioth E, Eldar Geva T, Levy-Lahad E, Renbaum P. Simultaneous preimplantation genetic diagnosis for Tay-Sachs and Gaucher disease. RBM Online. 2007;15:83–8. [DOI] [PubMed]
- 22.Obradors A, Fernández E, Oliver-Bonet M, Rius M, de la Fuente A, Wells D, et al. Birth of a healthy boy alter a double factor PGD in a couple carrying a genetic disease and at risk for aneuploidy: case report. Human Reprod. 2008;23:1949–56. [DOI] [PubMed]
- 23.Piyamongkol W, Vutyavanich T, Piyamongkol S, Wells D, Kunaviktikul C, Tongsong T, et al. A successful strategy for preimplantation genetic diagnosis of beta-Thalassemia and simultaneous detection of Down’s Syndrome using multiplex fluorescent PCR. J Med Assoc Thai. 2006;89:918–27. [PubMed]
- 24.Fiorentino F, Magli MC, Podini D, Ferraretti AP, Nuccitelli A, Vitale N, et al. The minisequencing method: an alternative strategy for preimplantation genetic diagnosis of single gene disorders. Mol Hum Reprod. 2003;9:399–410. [DOI] [PubMed]
- 25.Fiorentino F, Biricik A, Nuccitelli A, De Palma R, Kahraman S, Iacobelli M, et al. Strategies and clinical outcome of 250 cycles of preimplantation genetic diagnosis for single gene disorders. Hum Reprod. 2006;21:670–84. [DOI] [PubMed]