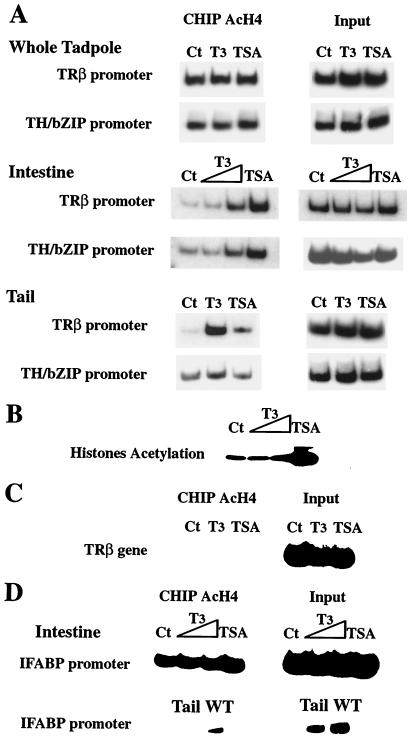
Figure 5.
T3 treatment increases histone H4 acetylation specifically at the TRE regions of T3 response genes in premetamorphic tadpole intestine and tail. (A) Organ-specific changes in histone acetylation by T3 and TSA. Stage 55 tadpoles were treated for 2 days with T3 (10 nM) or TSA (100 nM). Nuclei extract from whole tadpole, intestine, or tail were used for ChIP assay by using an antibody against acetylated histone H4. Aliquots of the chromatin before immunoprecipitation were used directly in PCR as a DNA control (input). (B) TSA but not T3 increases overall histone acetylation level in the intestine. Nuclear proteins (20 μg) from intestine of the above animals were loaded on a Tris-glycine 18% acrylamide gel (Novex). Proteins were transferred to a poly(vinylidene difluoride) Immobilon-P membrane (Millipore), and rabbit anti-acetyl-lysine polyclonal antibody (Upstate Biotechnology) was used to analyze core histone acetylation states. (C) T3 and TSA treatment has no effects on histone H4 acetylation in the transcribed region of TRβ gene far from the promoter in the intestine. ChIP assay was performed as in A for an internal region of TRβ gene instead of the promoter region. (D) Histone H4 acetylation levels at the promoter of IFABP gene, which is not a directly T3 response gene, in the intestine, tail, and whole tadpole (WT). Note that histone acetylation of the promoter could be detected in the intestine or whole tadpoles but not in the tail, where the IFABP gene is not expressed. TSA but not T3 treatment increased slightly histone H4 acetylation level at the promoter of IFABP gene in the intestine. All experiments were done at least twice.