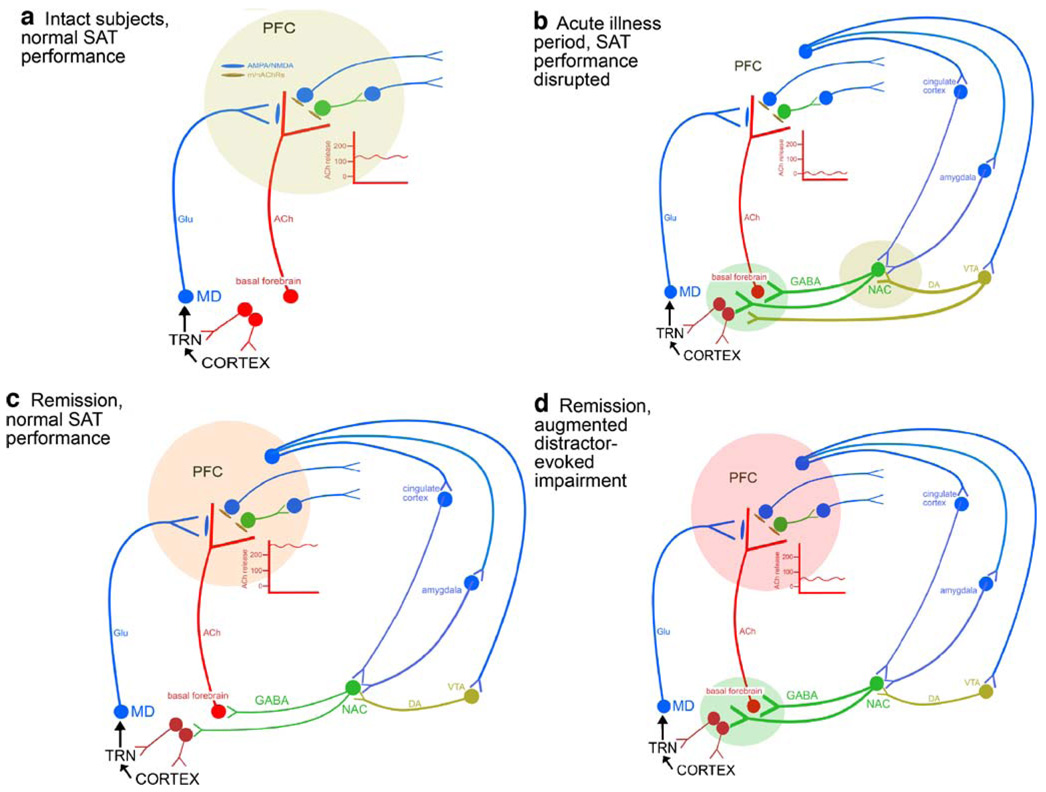
Fig. 4.
Circuitry models describing the regulation of the prefrontal (PFC) cholinergic input system mediating the performance of the standard sustained attention task (SAT) by healthy subjects (a), the disrupted SAT performance during acute illness periods (b), and the “normal” SAT performance (c) and the augmented detrimental effects of distractors during remission periods (d). The models explain key findings discussed in the main text and incorporate hypotheses concerning the mesolimbic regulation of prefrontal acetylcholine (ACh) release, as well as the abnormal top-down control of the basal forebrain and afferent mesolimbic systems during different disease states. a In intact subjects, standard SAT performance requires increases in PFC ACh release by about 100–140% over baseline (see insert); such increases in cholinergic activity specifically are required for the detection of signals (e.g., Parikh et al. 2007). As top-down control of the basal forebrain and its afferent mesolimbic systems [projections from prefrontal regions to the basal forebrain and to mesolimbic regions (nucleus accumbens, NAC, and ventral tegmentum, VTA), which in turn innervate the basal forebrain] is assumed to play an only minimal and perhaps negligible role for SAT performance by healthy subjects, standard-task performance-associated increases in PFC ACh release is thought to be based primarily on bottom-up mechanisms, meaning that the signal per se evokes cholinergic activity via local mechanisms in the PFC. We demonstrated that in the PFC, increases in cholinergic activity require glutamate (Glu) release and glutamatergic ionotropic receptor stimulation (Parikh et al. 2008). Such glutamate release originates from thalamic, mediodorsal (MD) afferents and is thought to “import” information about the signal to the PFC. This information does not concern the primary sensory representation of the signal but rather a signal-evoked “attentional searchlight”, a term that refers to a preattentional narrowing of the “place demanding attention”. The topographic projections from sensory cortical regions to the thalamic reticular thalamic nucleus (TRN), that in turn contacts MD neurons, underlie this preattentional processing of signals. The finding that attentional orienting is impaired following lesions of the TRN corresponds with this model (Weese et al. 1999). The TRN also receives cholinergic and non-cholinergic (not illustrated) inputs from the basal forebrain. The positive glutamatergic modulation of prefrontal cholinergic activity is necessary for normal signal detection and thus attentional performance. b As discussed in the main text, acute illness periods are associated with a severe disruption of attentional performance. The freezing of PFC ACh release at baseline causes such disruption. As the collective evidence is consistent with the hypothesis that mesolimbic dopaminergic (DA) activity is abnormally reactive during acute disease periods, and as NAC manipulations preferably affect PFC ACh release (e.g., Zmarowski et al. 2005, 2007), we hypothesize that basal forebrain cholinergic activity is robustly attenuated in part as a result of abnormally active dopaminergic afferents from the VTA and, indirectly, GABAergic afferents from the NAC. The basal forebrain may be further dysregulated by abnormal prefrontal output to mesolimbic regions and directly to the basal forebrain (not shown; Zaborszky et al. 1997). As a result of such freezing of basal forebrain activity, prefrontal ACh release remains at baseline and fails to support attentional performance. c During remission periods, standard (unchallenged) SAT performance is normal but mediated via abnormally high levels of PFC ACh release. The mechanisms underlying this finding remain unclear but are likely a result of abnormal levels of top-down control of the basal forebrain and its afferent mesolimbic systems (note that in contrast to the scenario described in b, in c such abnormal top-down activity does not interact with a hyper-responsive mesolimbic dopamine system). As discussed in the text, levels of prefrontal ACh release correlate with demands for top-down control, consistent with the hypothesis that during periods of remission, standard SAT performance requires abnormally high levels of top-down control. d During remission periods, attentional performance is extremely vulnerable to additional demands for top-down control, such as the presentation of distractors. The severity of the distractor effect allows the prediction that performance-associated ACh release levels will be close to baseline, similar to the scenario in b; however, this aspect of the model requires substantiation. As even standard SAT performance requires top-down control during remission periods, the additional challenge by distractors is speculated to reveal the abnormal interplay between dysregulated prefrontal output systems and abnormally reactive mesolimbic systems, yielding a result that may approach the freezing of the basal forebrain similar to the scenario described in b. The severity of the distractor-induced impairment in attentional performance, in patients and animals modeling remission periods (see main text), is consistent with an attenuated responsivity of the prefrontal cholinergic input system