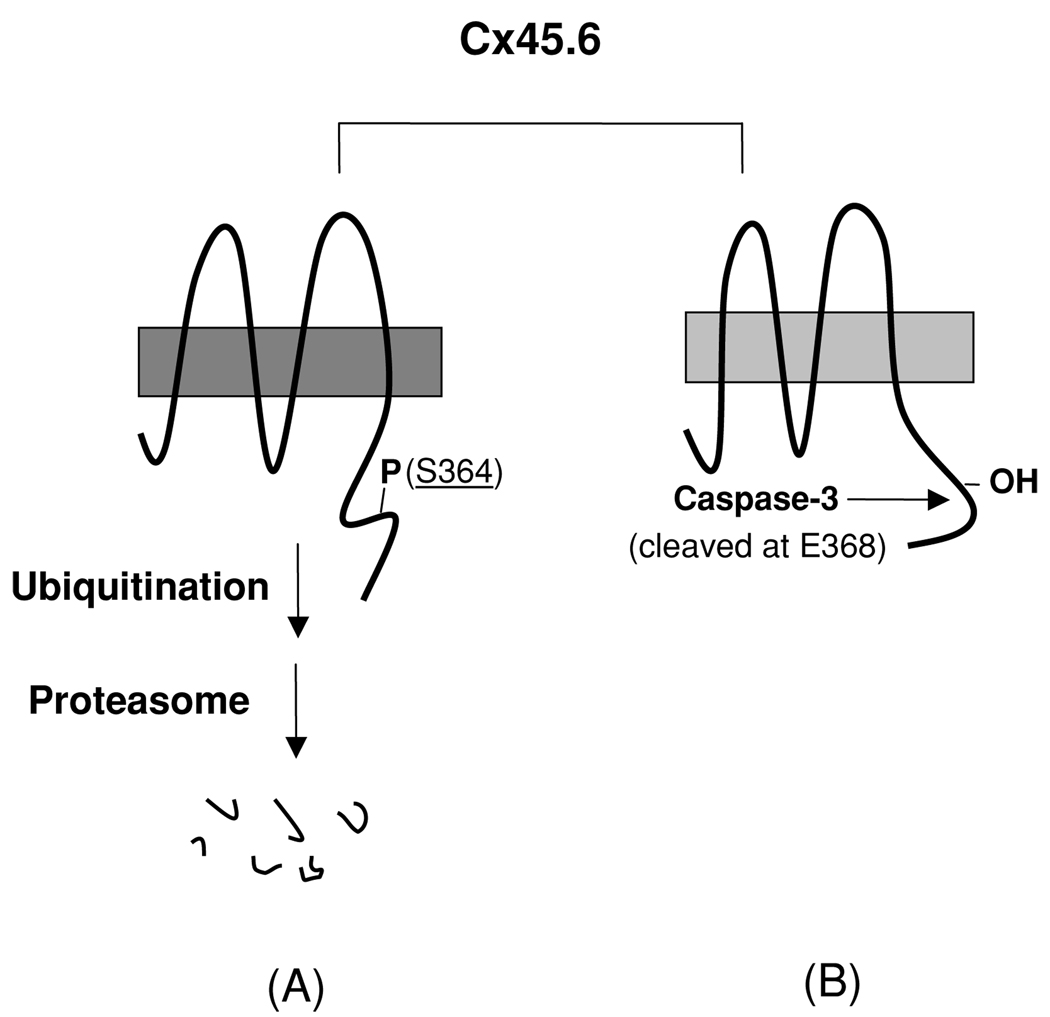
Figure 4.
A model for the regulation of proteolysis of Cx45.6 in the lens by phosphorylation at Ser364. (A) The phosphorylation at Ser364 renders Cx45.6 unstable and undergoes the degradation mediated by proteasome. (B) If Ser364 is not phosphorylated, Cx45.6 becomes a substrate for caspase-3, which cleaves behind Glu368. The truncated Cx45.6 mainly accumulates in the mature fibers at the center core region of the lens (Yin et al. 2001).