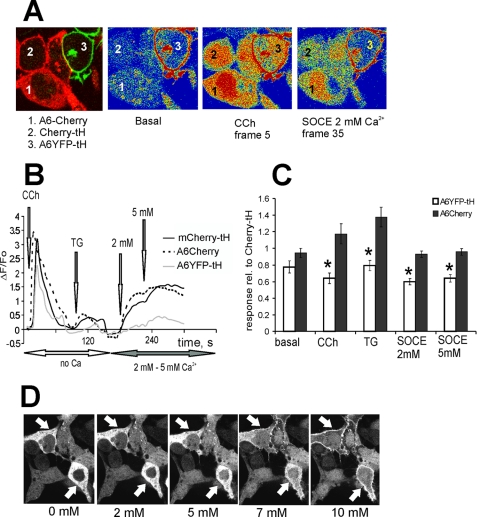
FIGURE 3.
A constitutive PM localization of annexin A6 is necessary for SOCE attenuation. A, HEK293 cells expressing annexin A6YFP-tH, wild-type cytoplasmic annexin A6-mCherry or mCherry-tH were co-cultured on the same coverslip, loaded with Fluo-3/AM, and examined in the confocal microscope. An arbitrary field containing all three cell types was selected, and changes in [Ca2+]i upon stimulation of intracellular Ca2+ release and store-operated Ca2+ entry examined by calcium imaging, selecting intracellular regions of interests to avoid the contribution of the YFP signal into Fluo-3 fluorescence changes observed in A6YFP-tH cells. B, cells were kept in Ca2+-free Tyrode buffer, stimulated with 10 μm CCh, followed by SERCA inhibition with 1 μm TG. SOCE was stimulated by adding 2 mm and 5 mm [Ca2+]ex, and responses in mCherry-tH-expressing cells (control, solid black line) were compared with annexin A6YFP-tK cells (gray line) and A6-mCherry (dotted black line). C, basal [Ca2+]i levels, CCh- and TG-stimulated ER release and Ca2+ entry in annexin A6-mCherry, and A6YFP-tH cells were expressed relative to responses of the control, mCherry-tH cells. The graph shows an average of three independent experiments (n = 9 arbitrary fields measured) ± S.E. SOCE was reduced to ∼60% of control levels in annexin A6YFP-tH construct (*, p < 0.05), but there was no statistically significant difference between the responses recorded in A6-mCherry cells and control, provided that annexin A6-mCherry remains in the cytoplasm. D, live HEK293 cells expressing annexin A6-mCherry (arrows) and mCherry were observed in the confocal microscope and treated with 1 μm TG followed by 2, 5, 7, and 10 mm [Ca2+]ex. Localization of annexin A6-mCherry after adding the indicated [Ca2+]ex is shown. There was no translocation of annexin A6-mCherry to PM at 2 or 5 mm [Ca2+]ex.