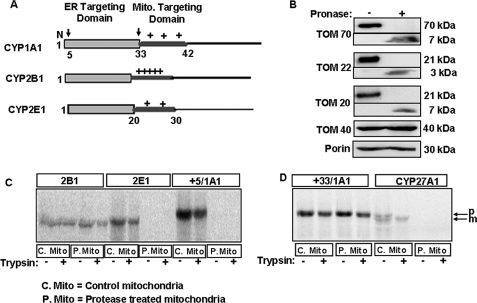
FIGURE 1.
Import of CYP proteins into protease-treated mitochondria. A, schematic representation of chimeric signals of CYP1A1, CYP2B1, and CYP2E1 with NH2-terminal ER-targeting domain followed by the positively charged mitochondrial (Mito.) targeting domains. Arrows over the CYP1A1 schematic indicate processing sites at the 4th and 32nd residues. B, isolated rat liver mitochondria were treated with or without Pronase as indicated under “Experimental Procedures.” The TOM components (full-length and fragments) were detected by immunoblot analysis (30 μg of protein each) using subunit-specific antibodies. C and D, 35S-labeled CYPs translated in a RRL system were used for import into Pronase-treated (P-mito) and untreated control (C-mito) mitochondria. The extent of import was determined by resistance to treatment with trypsin (200 μg/ml); mitochondria were pelleted through 1 m sucrose, and 250 μg of protein in each case was analyzed by SDS-PAGE on a 14% gel and subjected to fluorography. p and m indicate the precursor and mature forms of CYP27A1, respectively.