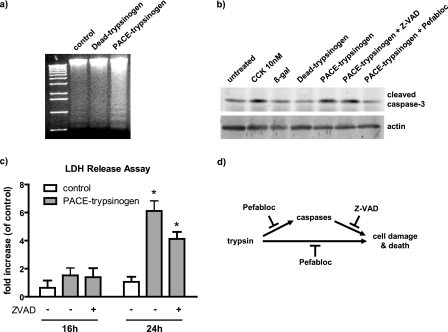
FIGURE 6.
Expression of PACE-trypsinogen caused acinar cell death. a, DNA fragmentation was elevated in PACE-trypsinogen virus-infected acinar cells compared with Dead-trypsinogen and β-galactosidase control virus-infected cells at 16 h postinfection. b, intracellular trypsin increased the level of cleaved caspase-3. Cells were infected with viruses bearing β-galactosidase, Dead-trypsinogen, or PACE-trypsinogen. After 16 h, cells were harvested, and proteins were blotted with an antibody specific for active caspase-3. PACE-trypsinogen-expressing acini showed increased caspase-3 cleavage, which could be blocked by Pefabloc (100 μm) but not by pancaspase inhibitor Z-VAD (50 μm). Acini treated with supraphysiologic cholecystokinin (10 nm) served as a positive control, and β-galactosidase- or Dead-trypsinogen-expressing virus-infected acini were used as negative controls. c, as a measure of more general cell damage, LDH release was evaluated in acini infected with PACE-trypsinogen virus at different times in the presence and absence of Z-VAD (50 μm). LDH release was not affected by 16 h after infection of acini. However, increased release of LDH was noted after 24 h, indicating advanced cell damage. This increase was not prevented by the presence of Z-VAD. Data presented show LDH -fold increase compared with untreated acini. Green fluorescent protein adenovirus-infected cells served as control. Results presented are means ± S.E. for three experiments. (*, p < 0.05 versus control). d, intracellular active trypsin caused cell death through caspase-dependent and -independent pathways. Inhibition of active trypsin with Pefabloc blocks both caspase activation and cell damage and death. However, inhibition of caspase activity with Z-VAD did not prevent cell death initiated by intracellular trypsin.