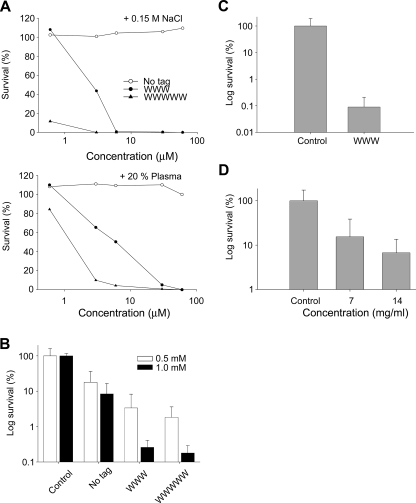
FIGURE 4.
Activities of peptides at physiological conditions, ex vivo, and in vivo. A, antibacterial effects of the indicated peptides against S. aureus in viable count assay. 2 × 106 CFU/ml of bacteria were incubated in 50 μl with the indicated peptides at 0.06–60 μm in 10 mm Tris, pH 7.4, 0.15 m NaCl (upper panel) or the same buffer containing 20% human plasma (lower panel). (The difference between the original peptide and its tagged variants is significant, p < 0.05, two-way ANOVA). B, activities of peptides in an ex vivo skin infection model. Pig skin was inoculated with S. aureus, and peptides at the indicated concentrations were added after an incubation time of 4 h. Bacteria were collected, and CFU was determined (mean values are presented; n = 6). Note the logarithmic scale on the y axis. (There is a statistically significant difference (p < 0.001 two-way ANOVA) between the tagged peptides versus control as well as the native peptide (no tag) at the two doses.) C, inoculation similar as in B, but pig skin was treated with GKH17 and GKH17-WWW (2.8 mm) in a polyethylene glycol formulation (PEG400/PEG3350/water 54/36/10). (There is a statistically significant difference between control treatment and WWW (p < 0.001, Student's t test).) D, treatment of S. aureus infected pig skin in vivo. Treatment with GKH17-WWW at two concentrations in a polyethylene glycol formulation (PEG400/PEG3350/water 54/36/10) and with formulation in the absence of peptide (2.8 and 5.6 mm). (There is a statistically significant difference between control treatment and the two concentrations of WWW (p < 0.05, one-way ANOVA).)