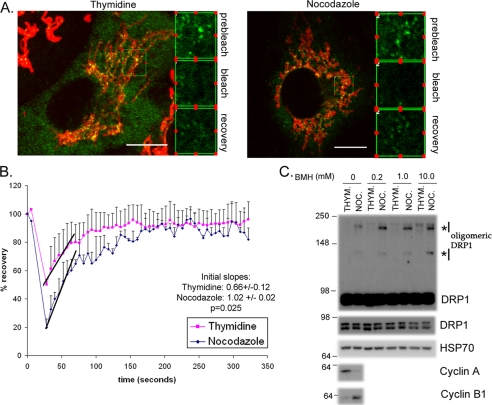
FIGURE 5.
DRP1 association with mitochondria is more labile, leading to an increase in functional oligomers during mitosis. A, HeLa cells transfected with DRP1-YFP were synchronized with thymidine or nocodazole. The nocodazole was washed out for 60 min to facilitate a more complete attachment of the cells to the coverslips during imaging. Cells were labeled with Mitofluor Red 633 and the extent of DRP1-YFP recovery after photobleaching is equivalent in both thymidine- and nocodazole-synchronized cells. B, the quantification of fluorescence recovery after the bleach shows that the rates of recovery are distinct. The average curves are shown from (n = 3) thymidine- and (n = 2) nocodazole-treated cells. The lines indicate the initial slopes of DRP1-YFP recovery following the bleach. Quantification of the slopes are indicated along with the statistical significance (inset). C, HeLa cells synchronized with thymidine or nocodazole were harvested, and the post-nuclear supernatants were incubated with increasing concentrations of bis(maleimido)hexane (BMH) cross-linker, as indicated. Western blots with anti-DRP1 antibodies reveal the enrichment of oligomeric forms of DRP1, which is specific to the nocodazole-treated cells (asterisks). HSP70 antibodies control for equal loading in each lane, where cyclins A and B indicate the efficiency of synchronization.