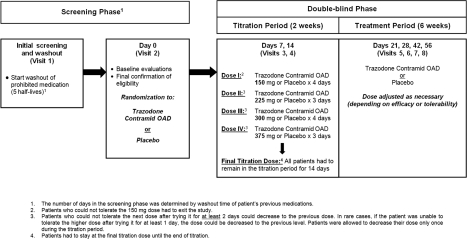
Figure 1.
- The number of days in the screening phase was determined by washout time of patient's previous medications.
- Patients who could not tolerate the 150 mg dose had to exit the study.
- Patients who could not tolerate the next dose after trying it for at least 2 days could decrease to the previous dose. In rare cases, if the patient was unable to tolerate the higher dose after trying it for at least 1 day, the dose could be decreased to the previous level. Patients were allowed to decrease their dose only once during the titration period.
- Patients had to stay at the final titration dose until the end of titration.