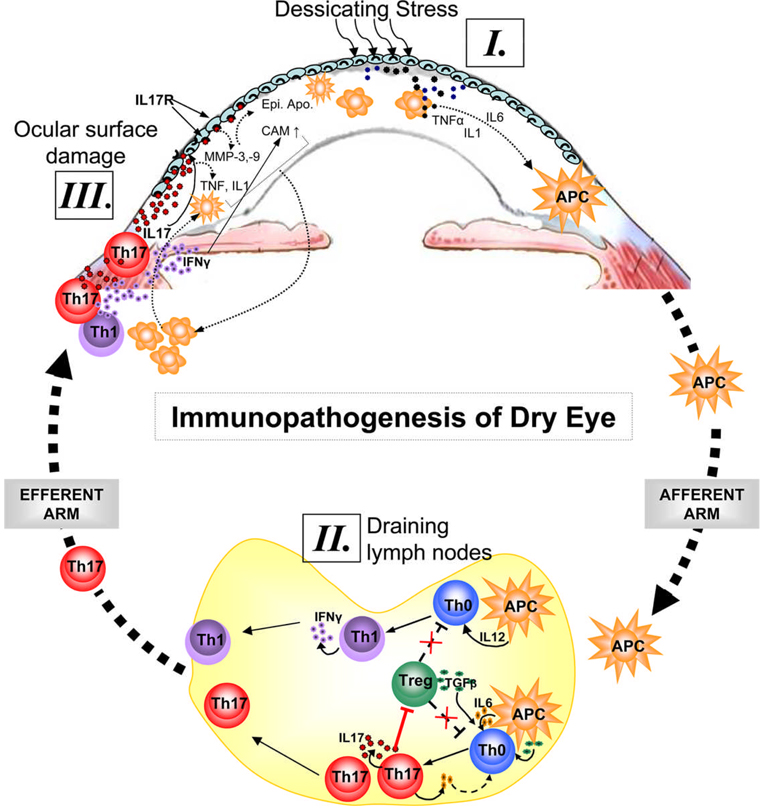
Figure 1. Immunopathogenesis of dry eye disease.
Ocular surface inflammation in DE is sustained by ongoing activation and infiltration of pathogenic immune cells, primarily CD4+ T cells in the conjunctiva and CD11b+ monocytic cells in the cornea1–5. [I.] Dessicating stress induces secretion of inflammatory cytokines especially IL-1, TNFα and IL-6 by ocular surface tissues which facilitate activation and migration of resident antigen presenting cells (APC) toward the regional draining lymph nodes (LN). [II.] In the LN, these APCs stimulate cognate naïve T cells (Th0) leading to the expansion of IL-17 secreting Th17 cells and IFN-γ secreting Th1 cells. IL-17 in turn antagonizes the Treg function by facilitating further expansion of Th17 cells which may compete with Tregs for TGF-β available in the milieu. [III.] Once these effectors are generated in the LN, they migrate to the ocular surface and secret effector cytokines. Interaction of IL-17 with its receptors on ocular surface leads to epithelial damage via increase secretion of matrix metalloproteinases (MMP) and inflammatory cytokines. In addition to apoptosis and metaplasia of ocular surface epithelia, IFN-γ causes upregulation of chemokine ligands/receptor and adhesion molecules (CAM) which facilitate increased ingress of immune cells to the ocular surface tissues.