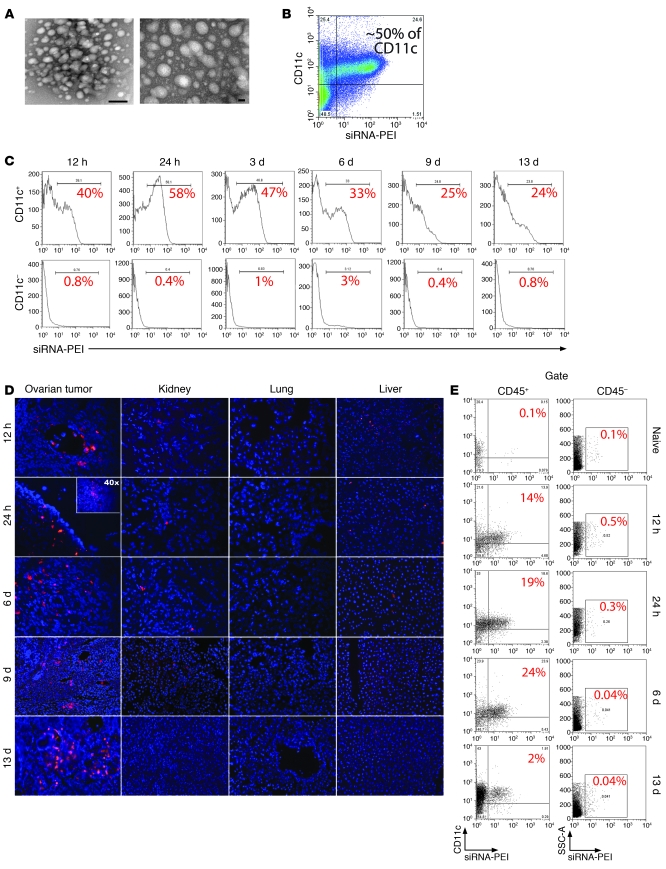
Figure 1. siRNA-PEI nanoparticles are preferentially engulfed by tumor-associated DCs.
(A) NTsiRNA-PEI were stained with uranyl acetate and visualized using transmission electron microscopy. Original magnification, ×145,000 (left); ×285,000 (right). Scale bars: 20 nm (left); 100 nm (right). Average nanoparticle size was 40–60 nm. (B) Selective engulfment of NTsiRNA-PEI by peritoneal tumor-associated CD11c+ DCs. Rhodamine-labeled NTsiRNA-PEI were intraperitoneally injected into mice bearing ID8-Defb29/Vegf-A ovarian carcinoma, and peritoneal wash samples were analyzed by FACS after 3 days. (C) Time-course analysis of nanocomplex uptake by peritoneal CD11c+ DCs in tumor-bearing mice after a single intraperitoneal injection. The percentage of cells retaining the nanoparticles is indicated for each time point. Data are representative of 3 mice analyzed per time point in 2 independent experiments. (D) Biodistribution of intraperitoneally injected siRNA-PEI nanoparticles. Ovarian carcinoma–bearing mice received a single intraperitoneal injection of rhodamine-labeled NTsiRNA-PEI, and multiple organs were collected at different time points after injection. Fluorescence microscopy was performed on histological sections from different organs to determine the presence of nanoparticles. Red indicates rhodamine-labeled nanocomplexes. Blue denotes nuclei. Data are representative of at least 3 independent experiments. Original magnification, ×200; ×40 (inset). (E) Nanoparticle uptake by DCs infiltrating solid ovarian tumors. Ovaries from tumor-bearing mice were collected at different time points after a single intraperitoneal injection with rhodamine-labeled NTsiRNA-PEI. Shown is the percentage of ovarian tumor–resident DCs engulfing nanoparticles in situ, determined by FACS. Data are representative of 2 independent experiments. SSC-A, side-scattered light.