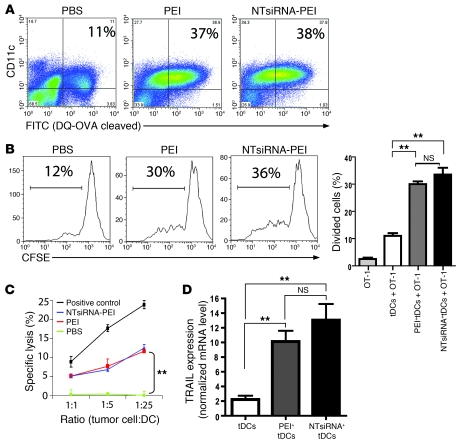
Figure 3. siRNA-PEI nanoparticles enhance antigen presentation and induce tumoricidal activity by ovarian cancer DCs.
(A) Improved antigen-processing capacity of tumor DCs engulfing PEI-based nanocomplexes in vivo. Mice bearing ID8-Defb29/Vegf-A tumors were intraperitoneally injected with DQ-OVA (see Methods), and 24 hours later received a single intraperitoneal injection of PBS, PEI, or NTsiRNA-PEI (3 mice per group, 2 independent experiments). Percentages denote the proportion of peritoneal tumor-associated DCs that efficiently processed the probe (FITC+), determined by FACS 48 hours later. (B) Enhanced antigen-presenting ability of OVA-pulsed tumor DCs (tDCs) engulfing siRNA-PEI nanoparticles in vivo (see Methods). Shown are representative FACS analysis of CFSE dilution and graphical representation of percent proliferating cells in triplicate for each condition. (C) Uptake of siRNA-PEI nanocomplexes induced tumoricidal DCs. Tumor-bearing mice were intraperitoneally injected with PBS, PEI, or NTsiRNA-PEI; 36 hours later, CD11c+MHC-II+CD3–NK1.1– tumor DCs from each group were sorted and incubated with 51Cr-labeled ID8-Defb29/Vegf-A cells. Release of 51Cr was measured in cell supernatants 5 hours later. Radiolabeled tumor cells were also cocultured with C57BL/6 splenocytes preincubated with 1,000 U/ml IL-2 for 5 days as a positive control of lysis. (D) Quantification of TRAIL mRNA levels by real-time RT-PCR in the same tumor DCs as in C. Error bars in B–D denote SEM. **P < 0.01.