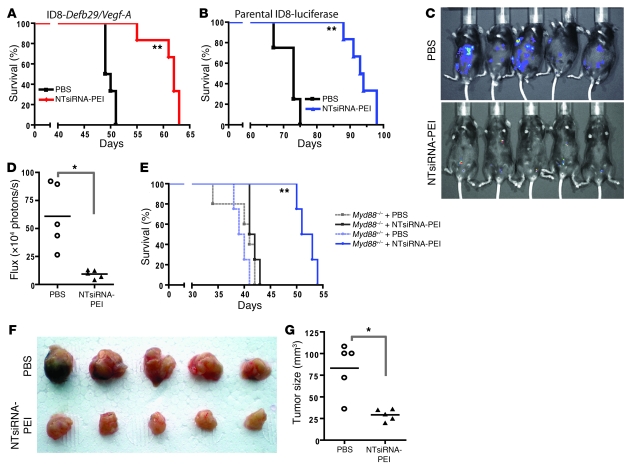
Figure 5. siRNA-PEI nanocomplexes prolong survival in a T cell–mediated, MyD88-dependent manner.
(A and B) Mice bearing aggressive ID8-Defb29/Vegf-A (A) or luciferase-expressing parental ID8 (B) tumors were treated intraperitoneally with PBS or NTsiRNA-PEI nanoparticles at days 8, 13, 18, 23, and 27 after tumor challenge, and survival was monitored over time. Data are representative of 2 independent experiments with a total of 12 mice per group. (C) Mice bearing preestablished luciferase-expressing parental ID8 ovarian tumors were intraperitoneally treated with PBS or NTsiRNA-PEI as described above, and luciferin was injected 70 days after tumor challenge to monitor tumor burden in vivo. (D) Quantification of the tumor burden shown in C. (E) Nanoparticle-mediated increase in survival was completely MyD88 dependent. Myd88+/– or Myd88–/– mice bearing aggressive ID8-Defb29/Vegf-A ovarian tumors were treated with PBS or NTsiRNA-PEI as described above, and survival was monitored over time. Data are representative of 2 independent experiments with a total of 10 mice per group. (F) Treatment with siRNA-PEI nanocomplexes induced T cell–mediated antitumor protective immunity. CD3+ T cells (3 × 106) purified from the spleens of mice treated with PBS or NTsiRNA-PEI were intravenously transferred into naive C57BL/6 mice previously irradiated with 3 Gy (n = 5 per group); after 24 hours, mice were challenged in the flank with ID8-Defb29/Vegf-A ovarian carcinoma cells. Tumor pictures were taken 2 months later. (G) Quantification of tumor size from F. In D and G, data points and horizontal bars denote individual values and means, respectively. *P < 0.05; **P < 0.01 versus respective PBS control, log-rank test (A, B, and E) or Mann-Whitney test (D and G).