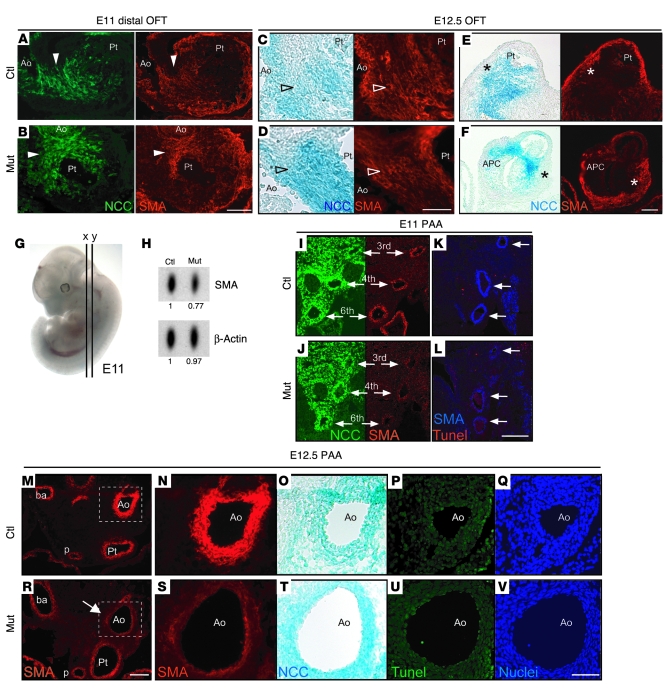
Figure 3. NCC differentiation in the cardiac outflow tract and aortic arch arteries of conditional Fak mutant embryos.
(A–F) Frontal sections of outflow tracts (OFT) stained for GFP and SMA. (A and B) At E11, NCCs expressing GFP are able to differentiate into smooth muscle in mutant embryos and control littermates (arrowheads). (C and D) At E12.5, NCCs (blue) differentiate into smooth muscle cells in the aorticopulmonary septum (arrowheads), even in mutants with abnormal aorticopulmonary communication. (E and F) Anterior sections from same embryos as in C and D, showing that myocardialization of the outflow tract in mutants is not altered (asterisks). At this stage, SMA stains for both smooth muscle and myocardium. (G) E11 embryo showing planes of sections for A and B (x) and I–L (y). (H) Western blot of E11 branchial arches (n = 3 embryos pooled), showing decreased SMA levels in mutants compared with control littermates. (I–V) Frontal sections through the pharyngeal arch arteries (PAA) stained for GFP, SMA, TUNEL, and DAPI. (I and J) At E11, NCCs colonize the third, fourth, and sixth pharyngeal arch arteries (arrows) in mutant and control littermates. (K and L) Reduction of SMA staining in mutant pharyngeal arch arteries (arrows) was not associated with increased NCC death. (M–V) E12.5 frontal sections showing preductal aortic arch region defective for NCC differentiation in a mutant (arrow). N–Q and S–V show higher magnification pictures from the boxed regions in M and R, respectively. O and T were stained with X-gal, and Q and V were stained with DAPI. p, pulmonary artery. Scale bars: 100 μm (A, B, E, F, M, and R); 50 μm (I–L, N–Q, and S–V); 25 μm (C and D).